Figure 3.
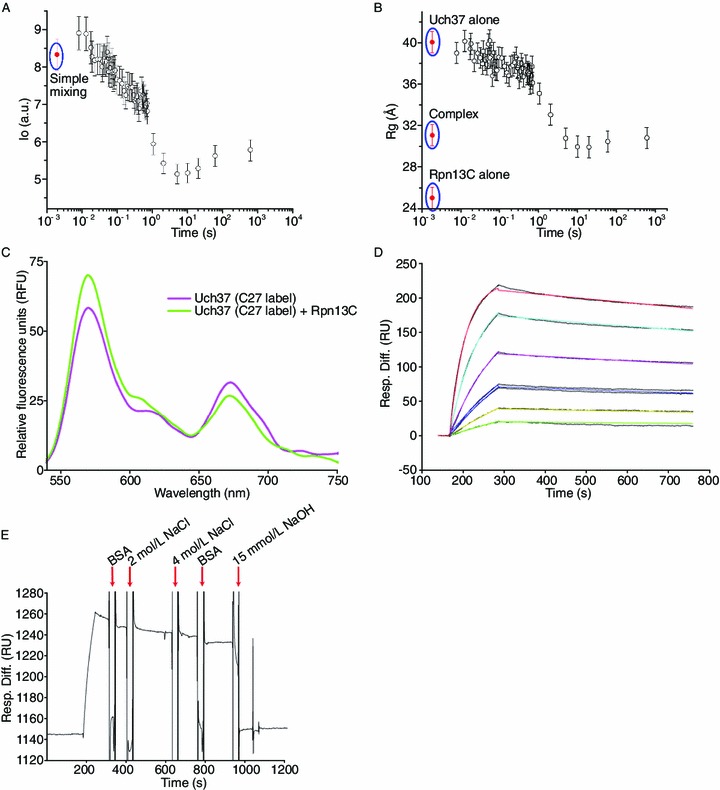
TR-SAXS, FRET, and SPR analysis of the Uch37-Rpn13C complex. (A) Graph of scattering intensity (I0) versus time and radius of gyration (Rg) versus time (B) during time-resolved SAXS analysis of a mixture of Uch37 and Rpn13C. Simple mixing represents the scenario where samples are assumed to be physically mixed without any chemical reaction taking place between the samples. The I0 and Rg values of Uch37 alone, Rpn13C alone, and the Uch37-Rpn13C complex are shown as red dots. The error bars represent the standard error of the mean of three independent measurements. (C) For the FRET experiments, three out of four cysteines present in Uch37 were mutated to alanine. Cys27 of Uch37 was conjugated to Alexa Fluor 555 (absorption maxima at 555 nm; emission maxima at 565 nm) and Alexa Fluor 647 (absorption maxima at 650 nm; emission maxima at 668 nm) (referred to as C27-label) randomly according to the manufacturer’s instructions. For all emission scans, the fluorophores in solution were excited at 550 nm, and their fluorescence emission was measured from 525 to 750 nm. The FRET experiment illustrating the decrease in the acceptor fluorescence at 664 nm and the corresponding increase in the donor fluorescence at 553 nm upon the addition of Rpn13C suggests reversal of the Uch37 oligomerization. (D, E) SPR analysis of the binding of Uch37 to Rpn13C. Rpn13C was immobilized, whereas a gradient of Uch37 was applied to quantify the binding affinity (panel D). Different colors indicate different concentrations of Uch37: green (15.6 nmol/L), yellow (31.2 nmol/L), blue (62.5 nmol/L), pink (125 nmol/L), cyan (250 nmol/L), and red (500 nmol/L). The binding was strong, and neither BSA (10 mg/mL) nor NaCl (4 mol/L) could break the complex. The proteins could be separated using 15 mmol/L NaOH (panel E)
