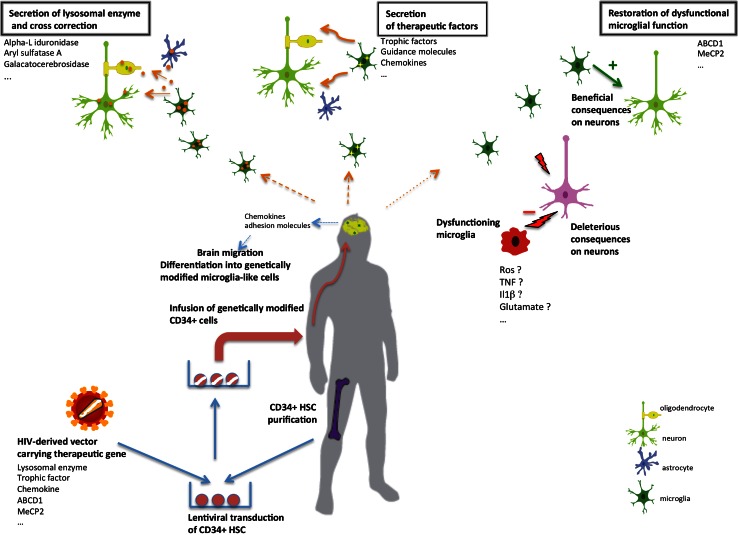
Fig. 2.
Transplantation of genetically modified hematopoietic stem cells to treat CNS diseases. After harvesting hematopoietic cells from the bone marrow of the patient, the CD34+ cell fraction containing hematopoietic stem cells (HSC) is purified and transduced with a lentiviral vector carrying the therapeutic gene. This gene can be the normal version of a deficient gene in patients with genetic diseases (ABDC1 in X-ALD, MeCP2 in Rett syndrome, lysosomal enzymes) or genes coding proteins of therapeutic interest like trophic factors, chemokines, and guidance molecules. Transduction with the lentiviral vector leads to stable integration of the therapeutic gene in the population of stem/progenitor cells. Genetically modified cells are re-infused into the patient and reconstitute the bone marrow stem cell compartment. A fraction of stem cells or myeloid progenitors is able to migrate to the CNS, cross the brain barrier and locally differentiate into genetically modified microglia-like cells. Chemokines (cell migration/chemotaxis inducing cytokines), such as monocyte chemoattractant protein 1 CCL2/MCP-1 and fractalkine CX3CL1/Fkn, produced by neurons, astrocytes and microglia, appear to attract peripheral blood mononuclear cells (PBMC) across the BBB into the brain parenchyma. Particularly, monocyte chemoattractant protein 1 (MCP-1) is and its receptor CCR2 have been implicated in a number of inflammatory diseases and are likely to be essential in this recruitment of bone marrow-derived cells. Migration of blood peripheral blood monocytic cells (PBMC) across the BBB into the brain parenchyma also depends on increased expression of various adhesion molecules, such as VCAM-1 ICAM-1, IG9 and E-selectin, all of which may promote PBMC attachment and transmigration. In the case of lysosomal diseases, lentivirus-mediated lysosomal enzyme overexpression allows efficient cross-correction of neurons and glial cells with diffusion of the therapeutic effect (left). HSC can also be engineered to express trophic factors with potential beneficial effects on surrounding neurons or glial cells (center). In diseases like X-ALD or Rett syndrome, the correction of deficient microglial function by transplantation of cells genetically modified with lentiviral vectors allows mitigation of neuronal damages induced by endogenous dysfunctioning microglia (right)