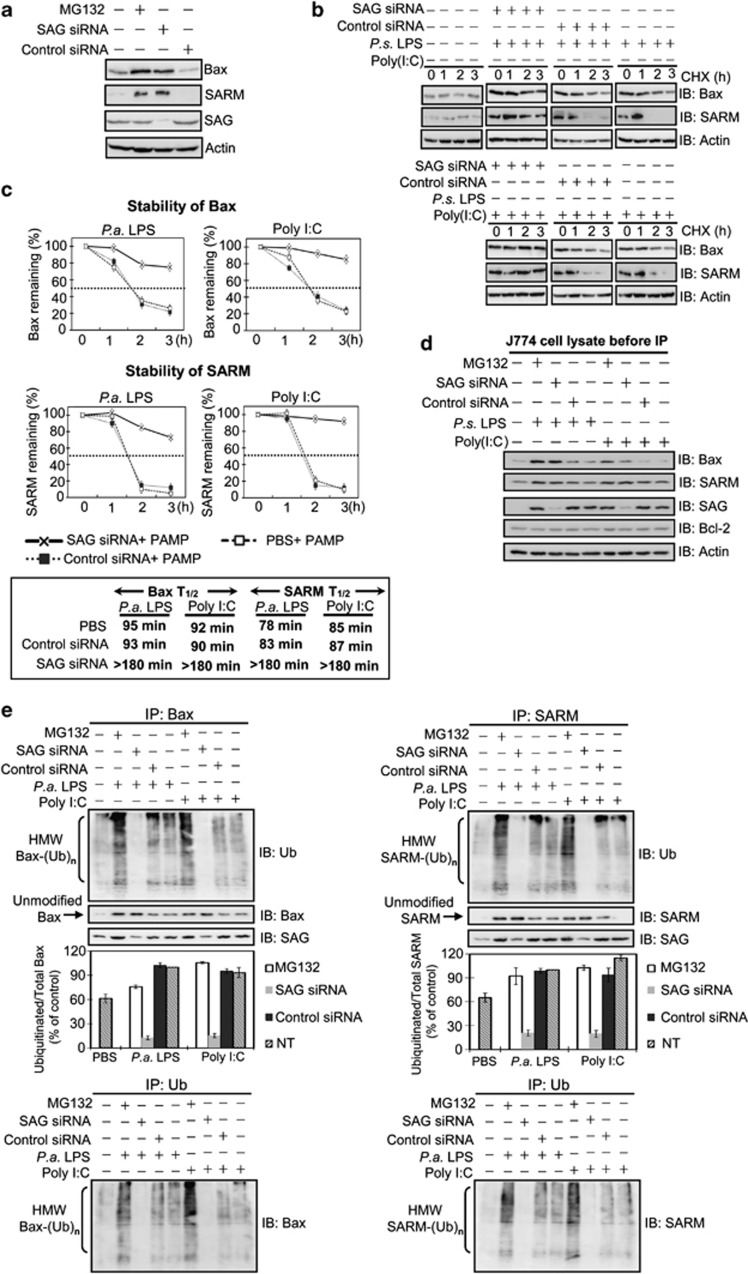
Figure 4.
SAG knockdown blocks ubiquitination and degradation of Bax and SARM, which accumulate in PAMP-treated macrophage J774 cells. (a) Cells were treated with MG132 or SAG siRNA to determine the involvement of SAG–proteasome system in the regulation of proapoptotic Bax and SARM. (b) The stability of Bax and SARM was tested in J774 cells with CHX, a protein synthesis inhibitor, and the lifespan of the proteins was examined by western blotting. (c) The half-lives (T1/2) of Bax and SARM were quantified by densitometry analysis. (d) Western blot analysis of whole-cell lysates shows the presence of Bax, SARM, SAG and Bcl-2 in the J774 cells (before IP). Actin was used as a loading control. (e) To investigate the association between SAG and Bax (or SARM) in infected cells, total cell lysates were immunoprecipitated with anti-Bax (or anti-SARM) antibody, separated by 12% and 10% SDS-PAGE, respectively, immunoblotted and probed with SAG, ubiquitin, Bax or SARM antibodies. SAG-knockdown attenuated the high-molecular-weight (HMW) modified forms of Bax and SARM (but not that of Bcl-2; see Supplementary Figure S6A). Arrows indicate unmodified (un-ubiquitinated) Bax or SARM. A densitometric analysis of ubiquitinated Bax or SARM, relative to total Bax or SARM is plotted (mean ±S.D., n=3). To confirm that the observed ubiquitination is due to Bax (or SARM) proteins, IP with ubiquitin, followed by immunoblotting with Bax (or SARM) was performed. IP with control rabbit IgG demonstrates the specificity of the assay (Supplementary Figure S7A). IgG, immunoglobulin G; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis