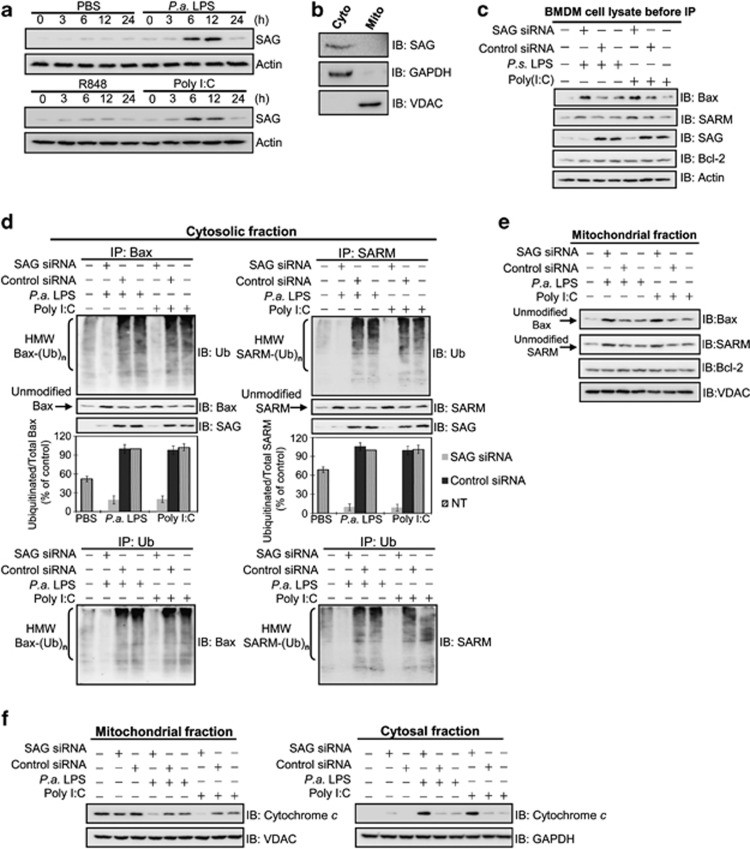
Figure 5.
SAG knockdown blocks ubiquitination of Bax and SARM in the cytosol of primary BMDM cells, with increased cytochrome c release. (a) The induction of SAG in infected BMDM was immunodetected. (b) Cells were pretreated with , polyinosinic-polycytidylic acid (poly I:C) (10 μg/ml) for 12 h, followed by cellular fractionation and western blotting. SAG is primarily expressed in the cytosol. (c) Western blot analysis of whole-cell lysates shows the presence of Bax, SARM, SAG and Bcl-2 in primary BMDM cells (before IP). The role of SAG in the ubiquitination of proapoptotic Bax or SARM was determined by Co-IP of subcellular fractions. Cells were treated with SAG siRNA, small interfering RNA (siRNA) (or control siRNA) for 16 h, followed by PAMP stimulation for 24 h. (d) Cytosolic fraction and (e) mitochondrial fraction. Both SAG-Bax and SAG-SARM immunoprecipitates were found in the cytosolic fraction. A densitometric analysis of the ubiquitinated Bax and SARM relative to total Bax and SARM in the cytosol is provided. Data are representative of means±S.D. (n=3). To confirm that the observed ubiquitination is due to Bax (or SARM) proteins, the IP with ubiquitin, followed by immunoblotting with Bax (or SARM) was performed. IP with control rabbit IgG shows the specificity of the assay (Supplementary Figure S7B). (f) Cytochrome c release was examined to determine the levels of intrinsic apoptosis, which is increased in SAG-knockdown BMDM.