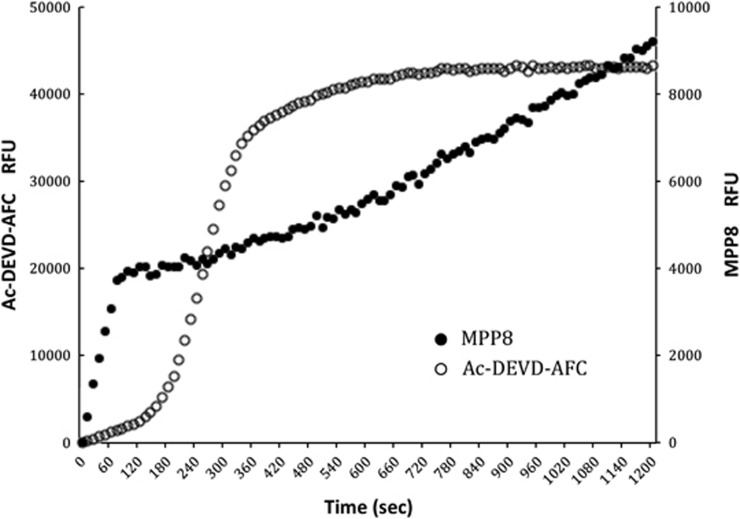
Figure 4.
Caspase activation time course. Cytosolic extracts were treated with cytochrome c and caspase activity was monitored simultaneously at different wavelengths by the caspase-9-selective substrate MPP8 that initiates after ∼1 min of addition of cytochrome c, whereas activity reported by Ac-DEVD-AFC exhibits a lag of ∼2–3 min before full activity is obtained. Eventually, Ac-DEVD-AFC substrate is depleted (∼6 min) and the curve relaxes to full substrate depletion. Because MPP8 is cleaved more slowly than Ac-DEVD-AFC, substrate is not depleted within the time course. The ‘burst' in the first 40–60 s is because of the interaction of the ACC substrate with cytochrome c through a mechanism that we do not yet understand, but is independent of any proteolytic activity, as it is observed in controls and is not observed for the AFC substrate. All substrates were used at 250 μM, and we could detect activity at 50 μM with the spectrofluorimeter employed in this study