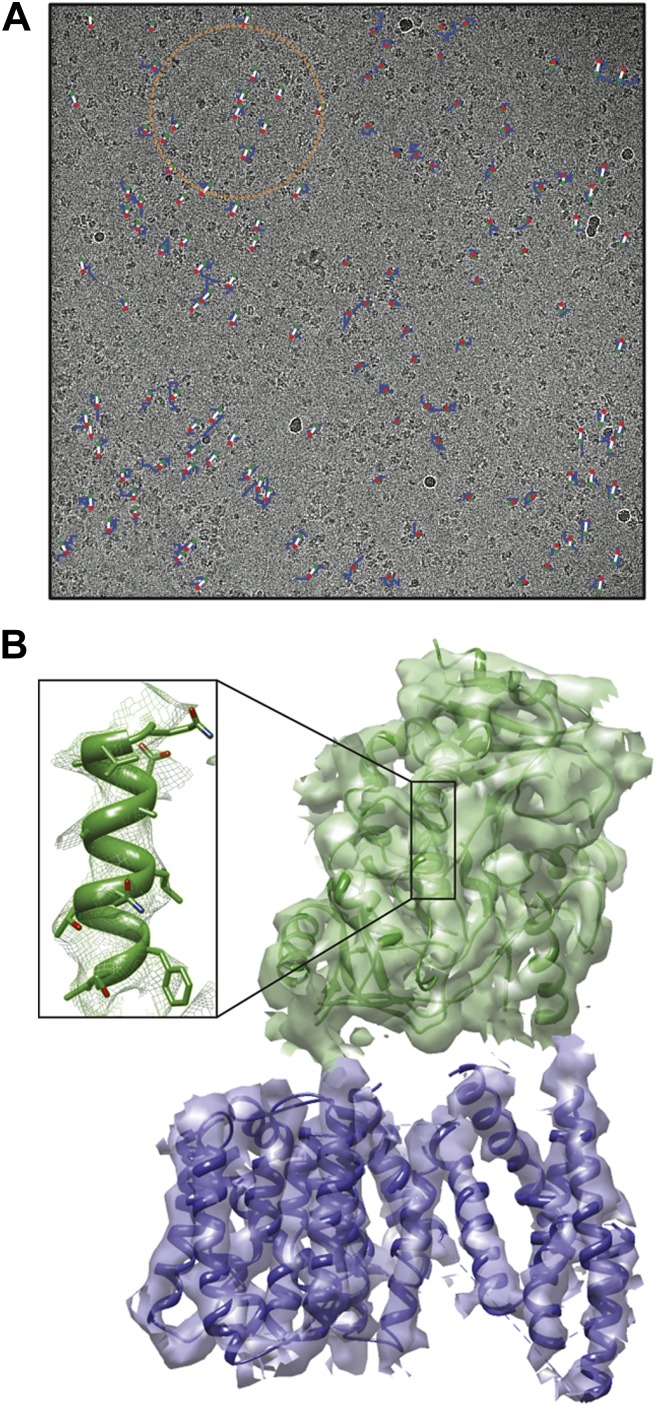
Figure 1. Cryo-EM structure of the human γ-secretase complex.
(A) Electron micrograph showing how the γ-secretase particles are moved by the electron beam (blue tracks; movement multiplied by a factor of 50 to make it visible). Scheres and co-workers have developed techniques (Bai et al., 2013; Scheres, 2014) to correct for these movements, and used them to determine the structure of the human γ-secretase complex at a resolution of 4.5 Å (Lu et al., 2014). This approach involves fitting linear tracks to the real movements: the fitted tracks are shown in white, with their start and end points being shown in green and red, respectively; the orange circle outlines the ensemble of particles used for statistical processing to fit the track of one particle (Scheres, 2014). (B) 3D map of the γ-secretase complex. The 19 trans-membrane helices of the four subunits that contain the active site of the complex are shown in blue, and the extracellular domain is shown in green. The inset shows an alpha helix with partly resolved sidechains in the extracellular domain.
CREDIT Scheres.