Figure 2.
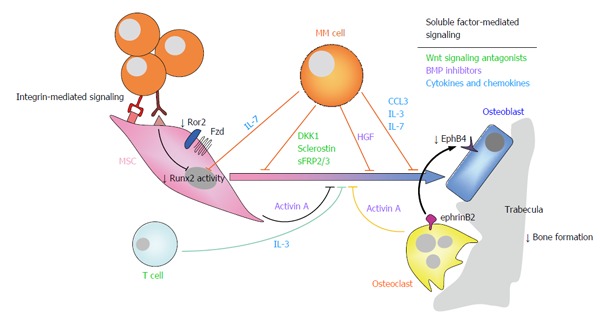
Suppression of osteoblastogenesis and osteoblast function in multiple myeloma is also involved in the pathophysiology of myeloma bone disease. Myeloma-induced OB suppression is partially mediated by direct cell to cell contact interactions with MSCs, leading to reduced activity of the Runx2/Cbfa1 transcription factor, and to inhibition of non-canonical Wnt5a signaling due to decreased expression of Ror2 in pre-OBs. In addition, soluble factors produced by myeloma cells and cells in the bone marrow microenvironment, such as Wnt signaling antagonists (e.g., DKK1, sclerostin, sFRP-2/3), BMP inhibitors (activin A, TGFβ, HGF), cytokines and chemokines (such as IL-7, TNFα, IL-3, CCL3) and apoptotic factors also contributed to inhibition of osteogenic differentiation and function. Finally, reduced ephrinB2-EphB4 signaling (from OCs to OBs) because of diminished EphB4 expression in MSCs, further contributes to impaired OB differentiation. Ror2: Receptor tyrosine kinase-like orphan receptor 2; DKK1: Dickkopf-1; sFRP-2/3: Secreted frizzled related protein-2/3; TGFβ: Transforming growth factor β; HGF: Hepatocyte growth factor; IL-7/3: Interleukin 7/3; ephrinB2: ephrin-B2 ligand; EphB4: Eph receptor B4; MSCs: Mesenchymal stromal cells; OB: Osteoblast; OC: Osteoclast; CCL3/MIP1α: Macrophage inflammatory protein 1-α; TNFα: Tumor necrosis factor α; BMP: Bone morphogenetic protein; MM: Multiple myeloma.
