Fig. 5. Etanercept administration to actively inflamed 10-week-old Sh3bp2KI/KI mice does not rescue facial swelling.
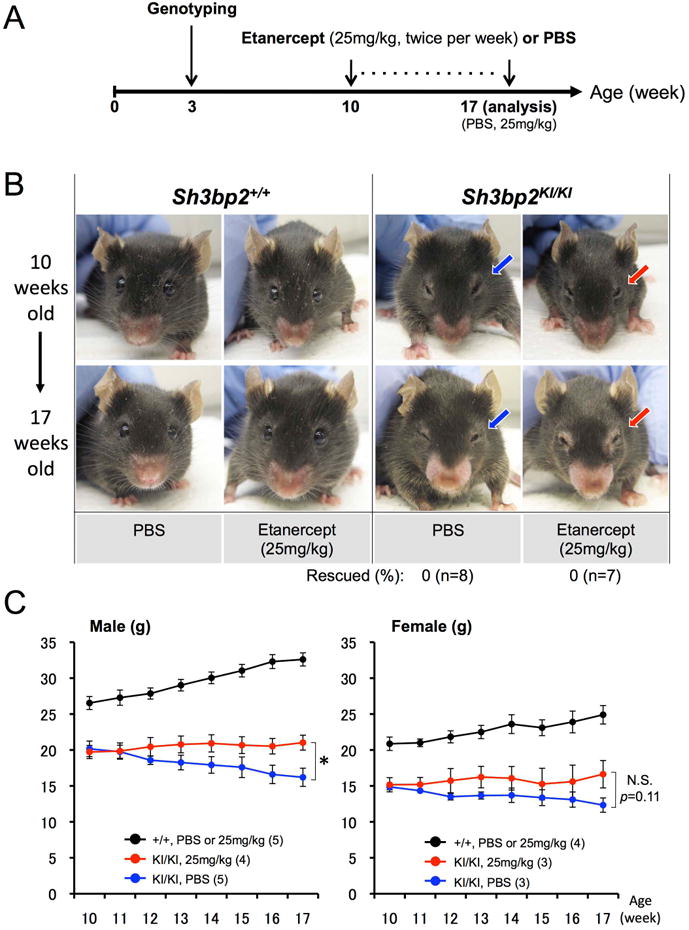
(A) Experimental procedure of etanercept administration to the 10-week-old Sh3bp2KI/KI mice with active inflammatory lesions. Ten-week-old Sh3bp2+/+ and homozygous Sh3bp2KI/KI mutant mice were treated with PBS or 25 mg/kg of etanercept twice per week for 7 weeks. Mice were sacrificed at 17 weeks of age for analysis. (B) Facial appearance of PBS- or etanercept-treated mice (top: before treatment at 10 weeks of age; bottom: after treatment at 17 weeks of age). Blue arrows indicate closed eyelids due to facial skin inflammation. Etanercept treatment failed to rescue eyelid closure in Sh3bp2KI/KI mice after etanercept administration (red arrows). Numbers represent the percentages of Sh3bp2KI/KI mice rescued from facial swelling. (C) Body weight changes in PBS- or etanercept-administered Sh3bp2+/+ and Sh3bp2KI/KI mice. Body weight in etanercept-administered Sh3bp2KI/KI mice (red line) was maintained, whereas weight of PBS-administered Sh3bp2KI/KI mice (blue line) continued to decrease. Numbers in parentheses represent the number of the mice weighed. Error bars represent ± SEM. Asterisk represents the significant difference (p < 0.05). N.S.: not significant.
