Fig 6. Natalizumab blocks the migration of T cells to the small intestine following teplizumab treatment.
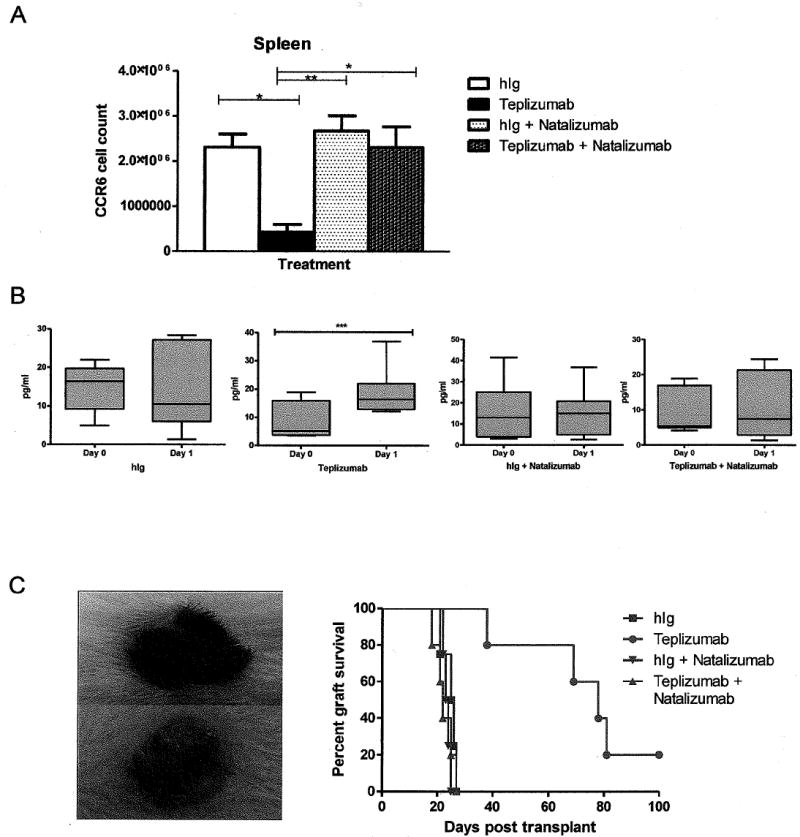
(A) The total number of CCR6+CD4+ splenocytes was determined by flow cytometry (n=8 mice/group). There was a significant decrease in the number of CCR6+CD4+ T cells after teplizumab treatment (*p<0.05) that was reversed with natalizumab treatment, the changes in CD4+CCR6+ cells were not significantly different when compared to teplizumab treatment (N=6) alone (p < 0.005). (B) The levels of IL-10 were measured in the serum of mice by Luminex, before and after treatment with teplizumab in the four treatment groups. There was a significant increase in the concentration of IL-10 only in the teplizumab group (*** p<0.001 by paired t-test, n=6-9/group, plots show median and 10th and 90th percentiles). Co treatment with natalizumab ablated this rise in IL-10. (C) Murine allogenic skin graft from B6 donor to reconstituted NSG mouse at day 15 following treatment with teplizumab (top) and hIg (below). The hIg treated mouse shows evidence of rejection. Co-treatment with natalizumab and teplizumab abated the treatment effect of teplizumab on graft survival. Each symbol represents an individual mouse (p=0.003 hIg v teplizumab, p=0.004 teplizumab v teplizumab + natalizumab).
