Figure 1. Optogenetic suppression of somatostatin-expressing (SOM) interneurons enhances gap detection.
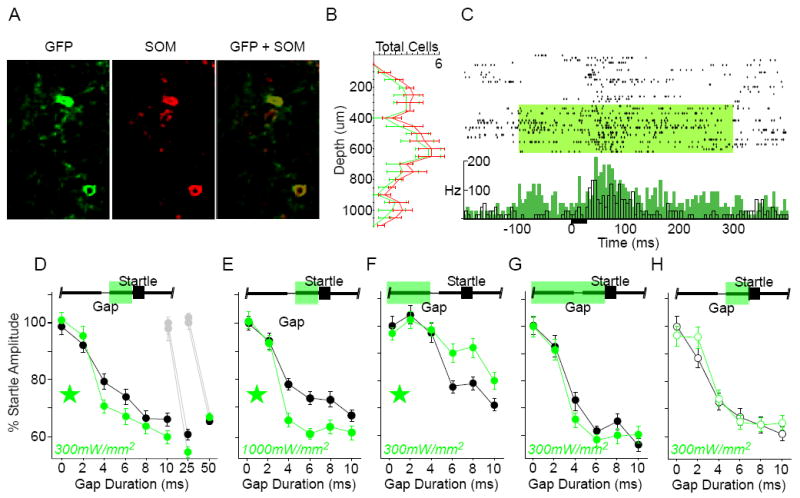
A) Co-localization of Arch-GFP native fluorescence (GFP) and somatostatin antibody labeling. 84% of somatostatin-positive cells expressed GFP (n = 4 sections, 2 mice). B) SOM interneurons were distributed throughout layers 2-6 in auditory cortex. The laminar distribution of GFP-labeled cells matched that of the overall somatostatin-positive population. Error bars show S.E.M. C) To confirm that activating Arch increased spiking activity in PNs, we recorded single-unit and multi-unit activity in anesthetized SOM mice. Illustrated is an example multi-unit recording from an anesthetized SOM animal. Top: raster plot of responses to a 25ms white noise burst (black bar), grouped by “laser off” and “laser on” trials (green shading: 300mW/mm2). Bottom: same data, shown as mean firing rate (black: “laser off”, green: “laser on”; 5ms bins). Suppressing SOM interneurons caused a significant increase in driven (0-100ms) and spontaneous (-100-0ms) spiking activity. Efficacy was verified in 4 mice. Suppressing SOM interneurons (300mW/mm2) significantly increased sound-evoked spiking activity for 9/23 (39%) recordings, 4 of which also showed a significant increase in spontaneous activity. Interestingly, most of these effects were seen in the subgranular layers: 75% of multi-unit sites deeper than 500μm showed significant effects of laser illumination. D-H) Gap detection behavior. The green bar at the top of each panel indicates the laser duration relative to Gap and Startle stimuli; green stars indicate significance within the ANOVA; error bars are S.E.M. D) Post-gap suppression, low intensity: Suppression of SOM interneurons specifically during the post-gap interval significantly attenuated startle responses (indicating enhanced gap detection) following gaps of ≤10ms [interaction F(5,1430) = 2.22, p = .049; 3 mice, 12 sessions] and 25ms (df = 718, t = 2.6, p = .009; 3 mice, 10 sessions), but not 50ms (4 mice, 12 sessions). Gray symbols correspond to the 0ms gap presentations during separate assessment of 25ms or 50ms gap detection. E) Post-gap suppression, high intensity: Increasing the laser intensity to 1000mW/mm2 further attenuated the startle following gaps ≤10ms [main effect F(1,206) = 14.98, p = .0001, and interaction F(5,1030) = 3.338, p = .006; 2 mice, 10 sessions]. F) Pre-gap suppression, low intensity: Suppression restricted to the pre-gap interval, beginning 1000ms prior to startle onset, enhanced startle responses to gaps ≤10ms [main effect F(1,234) = 4.71, p = .031, and interaction F(5,1170) = 3.37, p = .005; 4 mice, 10 sessions). G) Prolonged suppression, low intensity: Prolonged suppression, beginning 1000ms prior to startle onset and continuing through the post-gap interval, had no effect on gap detection (3 mice, 12 sessions). H) Gap detection was unaffected by the laser in Arch-negative SOM littermate controls (open circles; 3 mice, 10 sessions).
