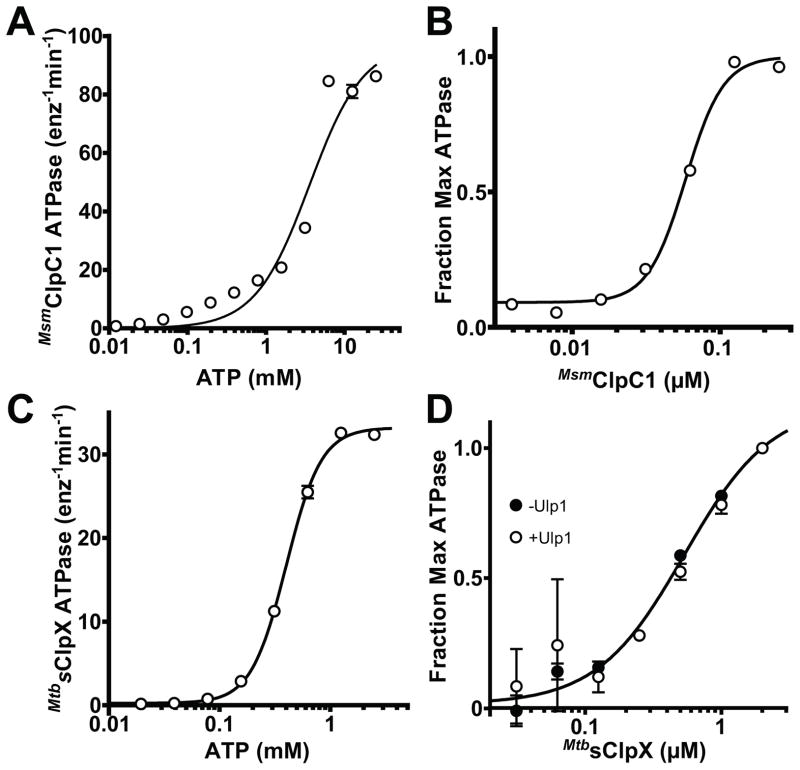
Figure 1. ATPase activity and hexamer stability of mycobacterial ClpX and ClpC1.
A MsmClpC1 (0.5 μM) ATPase activity was measured as a function of ATP concentration and fit to a Hill equation (apparent KM = 3.6 ± 0.6 mM; Hill n = 1.3 ± 0.2).
B Dependence of ATPase activity on MsmClpC1 concentration. The line is a fit to a Hill equation (K0.5 = 58 ± 1.9 nM; n = 3.4 ± 0.4).
C MtbsClpX (0.5 μM) ATPase activity was measured as a function of ATP concentration and fit to a Hill equation (apparent KM = 0.4 ± 0.006 mM; n = 2.7 ± 0.09).
D Dependence of ATPase activity on MtbsClpX concentration. The line is a fit to a Hill equation (K0.5 = 0.54 ± 0.09 μM; n = 1.4 ± 0.3 nM). Removal of the N-terminal SUMO domain from MtbsClpX by cleavage with the Ulp1 protease (confirmed by SDS-PAGE) had little effect on hexamer stability (K0.5 = 0.66 ± 0.23 μM; n = 1.8 ± 0.8).
Values in all panels are averages (N = 3) ± 1 standard deviation (SD).