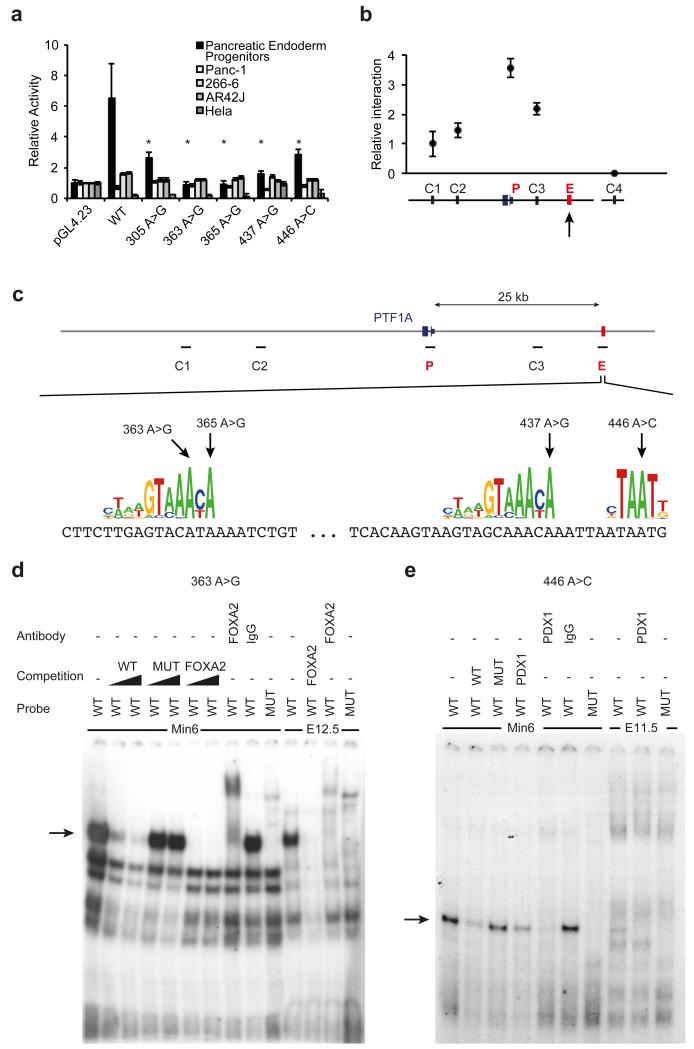
Figure 3. Pancreas agenesis mutations disrupt the function of a transcriptional enhancer that is specifically active in pancreatic progenitors.
(A) Reporter assays for the novel enhancer in hESC-derived pancreatic endoderm progenitors, several adult pancreatic exocrine transformed cells (Panc-1, 266-6, AR42J) and HeLa cells. Transcriptional enhancer activity was only observed in hESC-derived pancreatic progenitor cells, and it was disrupted by all 5 mutations. Asterisks indicate Student’s t test P<0.05 for comparisons of mutant vs. wild type enhancers in progenitors. (B) Chromosome conformation capture shows that the newly identified enhancer interacts directly with PTF1A promoter in hES cell-derived pancreatic progenitor cells. The viewpoint at PTF1A is signaled with an arrow, and the approximate regions that were tested for interaction with the viewpoint (C1, C2, C3, and P, for PTF1A promoter) are shown in (C). C4 represents a control region in an unrelated locus. (C) Pancreas agenesis mutations target critical residues in predicted binding sites for pancreatic regulators FOXA2 and PDX1. (D) Electrophoretic mobility shift assay showing high affinity, sequence-specific interaction of FOXA2 with a double stranded oligonucleotide containing the wild type (WT) sequence, but not the 363 A>G mutation (MUT). The retardation signal was suppressed by unlabelled consensus high-affinity binding site for FOXA2, and supershifted with antibodies recognizing FOXA2. Black triangles represent competition gradients of 30 and 100-fold cold probe excess. Binding activity is shown for MIN6 cells and dissected pancreatic buds from e12.5 mouse embryos. Probe refers to the radioactively labelled probed used in binding assays. (E) Electrophoretic mobility shift assay showing sequence-specific interaction of PDX1 with oligonucleotide containing the wild type (WT) sequence, but not the 446 A>C mutation (MUT). The retardation signal was suppressed by unlabelled consensus high-affinity binding site for PDX1, and supershifted with antibodies recognizing PDX1. Competition was performed with 100-fold cold probe excess. Binding activity is shown for MIN6 cells and pancreatic buds from e11.5 mouse embryos.