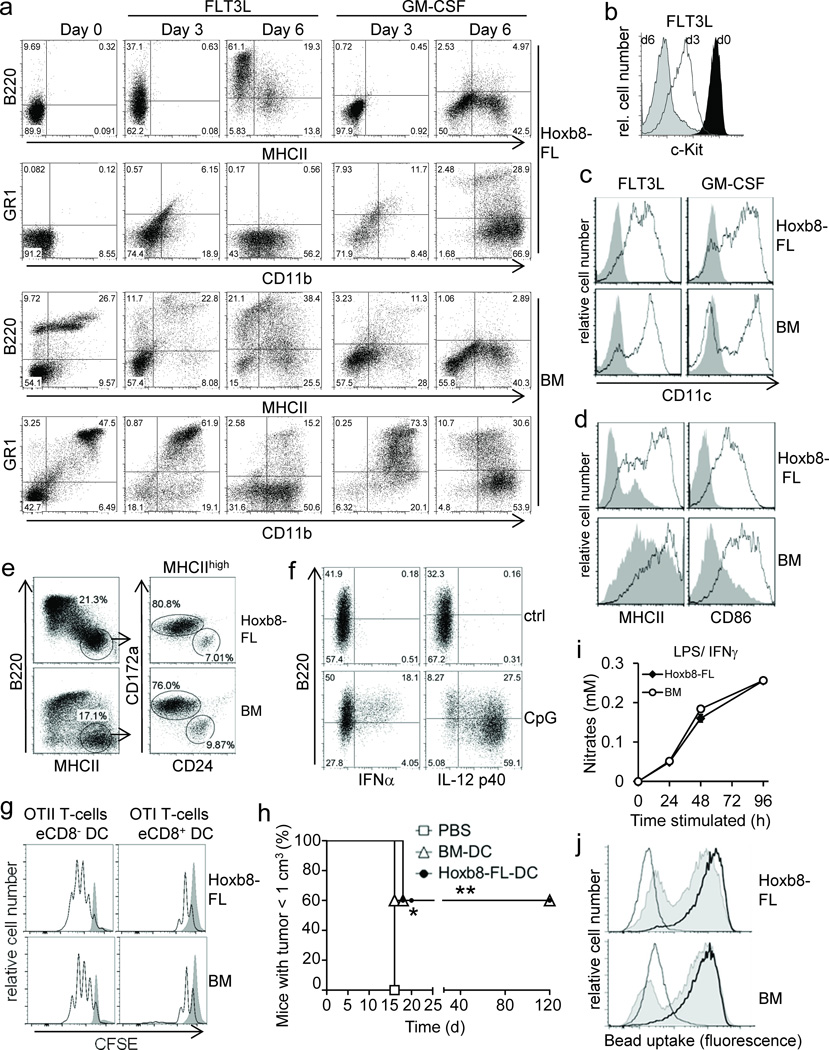
Figure 2. Phenotype and function of Hoxb8–FL– and BM–derived myeloid cells.
(a–c) Hoxb8–FL cells or BM cells were cultured in the presence of FLT3L or GM–CSF and analyzed after three and six days (a,b) and six days (c) by flow cytometry. Day 0 corresponds to cells prior to differentiation. (d) Cells were differentiated with FLT3L for six days, followed by treatment with (open) or without (filled) CpG–DNA (1668) for 18 hours and flow cytometry analysis. (e) Cells were differentiated with FLT3L for ten days, followed by flow cytometry analysis. (f) Hoxb8–FL cells were differentiated with FLT3L for six days, treated with or without (ctrl) CpG–DNA (2216; CpG), followed by intracellular staining for IFNα and IL–12p40 and flow cytometry. (g) Cells were differentiated with FLT3L for nine days. Sorted eCD8+ DCs and eCD8– DCs were pulsed with OVA and co–incubated with CFSE–labeled naïve CD4 and CD8 T–cells from TCR–transgenic OTII and OTI mice. T–cell proliferation in the absence (filled) or presence of DC (open) was analyzed by flow cytometry after 72 hours. (h) Cells were differentiated with GM–CSF for nine days, pulsed with OVA for 4 hours and stimulated with CpG–DNA for 18 hours. Mice were immunized with cells or PBS subcutaneously and challenged one week later intra–dermally by OVA–expressing B16 cells. Mice were sacrificed when tumor size reached 1 cm3. n=5 mice per group. * = P < 0.05; ** = P < 0.003 (log–rank test). (d) Cells were differentiated with M–CSF, treated with LPS and IFNγ, and Nitrate levels in supernatants were determined. Error bars represent standard deviation of three biological replicates. (j) Cells were differentiated with M–CSF, incubated with FITC–labeled IgG–coated beads at 37°C for 2 h (solid) or 6 h (thick line), or at 4°C for 6 h (thin line), followed by flow cytometry analysis.