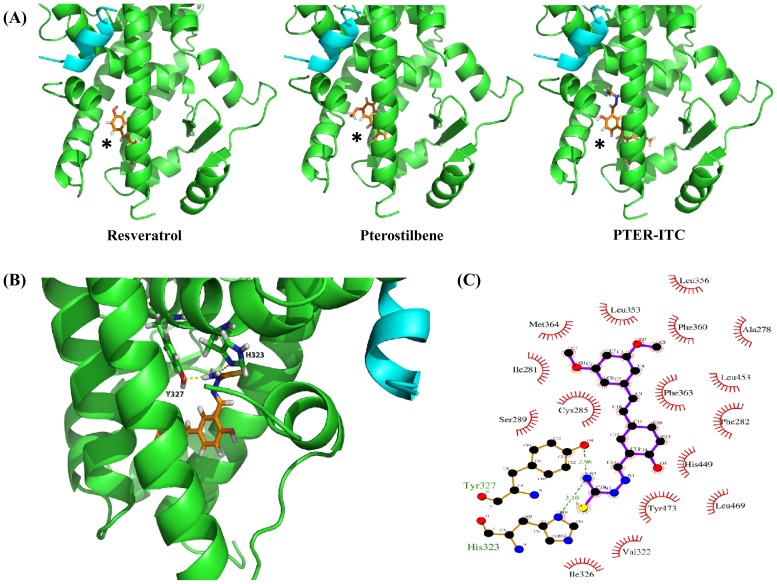
Figure 5. Analysis of PTER-ITC docking pattern with PPARγ.
(A) Mode of binding of resveratrol, PTER and PTER-ITC to PPARγ. Note the distinct orientations of the ligands. The broad range of ligand binding ability of PPARγ can be explained in part by the large T-shaped ligand binding area, which permits ligands to adopt distinct orientations (figures generated with PyMOL molecular graphics system). (B) Interaction of PTER-ITC within the ligand-binding pocket. Residues H323 and Y327 of protein chain A are involved in hydrogen bond formation with N3 of the ligand. Yellow dashed lines indicate bonding; interacting residues are labeled. (C) Ligand interaction plot showing different hydrophobic and two hydrogen bond interactions of PTER-ITC with PPARγ. Hydrogen bonds are indicated by green dashed lines, with their respective distances.