Abstract
The β1-adrenoceptor exists in two agonist conformations/states: 1) a high-affinity state where responses to catecholamines and other agonists (e.g., cimaterol) are potently inhibited by β1-adrenoceptor antagonists, and 2) a low-affinity secondary conformation where agonist responses, particularly CGP12177 [(−)-4-(3-tert-butylamino-2-hydroxypropoxy)-benzimidazol-2-one] are relatively resistant to inhibition by β1-adrenoceptor antagonists. Although both states have been demonstrated in many species (including human), the precise nature of the secondary state is unknown and does not occur in the closely related β2-adrenoceptor. Here, using site-directed mutagenesis and functional measurements of production of a cyclic AMP response element upstream of a secreted placental alkaline phosphatase reporter gene and accumulation of 3H-cAMP, we examined the pharmacological consequences of swapping transmembrane (TM) regions of the human β1- and β2-adrenoceptors, followed by single point mutations, to determine the key residues involved in the β1-adrenoceptor secondary conformation. We found that TM4 (particularly amino acids L195 and W199) had a major role in the generation of the secondary β1-adrenoceptor conformation. Thus, unlike at the human β1-wild-type adrenoceptor, at β1-TM4 mutant receptors, cimaterol and CGP12177 responses were both potently inhibited by antagonists. CGP12177 acted as a simple partial agonist with similar KB and EC50 values in the β1-TM4 but not β1-wild-type receptors. Furthermore pindolol switched from a biphasic concentration response at human β1-wild-type adrenoceptors to a monophasic concentration response in the β1-TM4 mutant receptors. Mutation of these amino acids to those found in the β2-adrenoceptor (L195Q and W199Y), or mutation of a single residue (W199D) in the human β1-adrenoceptor thus abolished this secondary conformation and created a β1-adrenoceptor with only one high-affinity agonist conformation.
Introduction
The β1-adrenoceptor exists in two agonist conformations or states. Catecholamines activate the primary, high-affinity, catecholamine conformation, and these responses are readily inhibited by β-blockers (β-adrenoceptor antagonists). Much higher concentrations of β-blockers are required to inhibit secondary conformation agonist responses (e.g., response to CGP12177 [(−)-4-(3-tert-butylamino-2-hydroxypropoxy)-benzimidazol-2-one]), and this conformation is therefore termed the secondary, low-affinity, CGP12177 conformation (Pak and Fishman, 1996; Granneman, 2001; Molenaar, 2003; Arch, 2004).
Conventional partial agonists need to saturate receptors to stimulate their maximum response and, thus, the concentrations required for half receptor occupancy (KB) should normally be equal to the concentration required to stimulate half maximum response (EC50) value. However, early observations found that several β-blockers with partial agonist activity did not conform to this. The concentration of pindolol, for example, needed for the cardiostimulant effects in feline heart was 10 times greater than that needed to antagonize responses of the full agonist isoprenaline and thus was labeled as a nonconventional partial agonist (Kaumann, 1973; Kaumann and Blinks, 1980; Kaumann and Molenaar, 2008). CGP12177 (Staehelin et al., 1983) was also found to be a nonconventional partial agonist, with even larger differences between KB and EC50 (Kaumann, 1983), and, therefore, it has become an important tool in understanding β1-adrenoceptor pharmacology (Kaumann and Molenaar, 2008). In 1996, Pak and Fishman (1996), using transfected cells, showed that CGP12177 inhibited isoprenaline responses at low concentrations (i.e., with high affinity), but required 100 times higher concentrations to stimulate responses. Furthermore, CGP12177 agonist responses required higher concentrations of conventional β-blockers to antagonize them. They therefore proposed a high-affinity and a low-affinity state or conformation of the β1-adrenoceptor, and this terminology has now been widely adopted (Granneman, 2001; Kaumann et al., 2001).
Studies using transfected cells have determined the site of action of many β-ligands (e.g., Konkar et al., 2000; Baker et al., 2003; Joseph et al., 2004a; Baker, 2005a). Pindolol, alprenolol, and several other ligands have biphasic concentration-response relationships (Walter et al., 1984; Baker et al., 2003; Kaumann and Molenaar, 2008; Baker, 2010a). The high-affinity component (readily blocked by β-blockers) is thought to occur via the high-affinity catecholamine conformation, while the low-affinity component (resistant to β-blockade) occurs via the secondary low-affinity conformation (Walter et al., 1984; Baker et al., 2003).
These two β1-adrenoceptor conformations have also been demonstrated in rat, mouse, ferret, and human heart (e.g., Kompa and Summers, 1999; Kaumann et al., 2001; Lowe et al., 2002; Joseph et al., 2003; Sarsero et al., 2003; Molenaar et al., 2007), human blood vessels (e.g., Mallem et al., 2004; Kozlowska et al., 2003, 2006), and in whole animals (e.g., Malinowska and Schlicker, 1996; Zakrzeska et al., 2005). However, the physiologic or clinical relevance of this conformation remains unknown, even though the plasma concentration of carvedilol (100 ng/ml = 300 nM) used in human cardiovascular diseases is sufficient to occupy this secondary conformation (Sawangkoon et al., 2000; Baker et al., 2003).
Limited mutagenesis studies have, in passing, noted secondary conformation effects and detected some overlap of the amino acids involved in the catecholamine conformation, although these studies are not all in agreement. D138 (transmembrane [TM] 3) and N363 (TM7) have been suggested by some (Baker et al., 2008), but not all (Joseph et al., 2004b) to be involved in the secondary conformation, as have mutations in TM5 (Kaumann and Molenaar, 2008) and TM6 (Baker et al., 2008). However, the precise nature of this secondary conformation remains unknown from its location within the receptor sequence to the physiologic relevance or potential clinical implications (Molenaar, 2003; Molenaar et al., 2007).
Finally, although the β1-adrenoceptor of many species (rat, mouse, guinea pig, ferret, cat, and human) (Kaumann and Molenaar, 2008), and the human β3-adrenoceptor (Baker, 2005b) have been shown to exist in at least two agonist conformations, this is not true for all β-adrenoceptors. CGP12177 is a conventional partial agonist at the human β2 and turkey β3C adrenoceptors, (i.e., with similar KB values and EC50 values) suggesting interaction via a single conformation (Pak and Fishman, 1996; Baker et al., 2002; Baker, 2010b).
This study aimed to discover the primary region important for the secondary conformation of the β1-adrenoceptor. Using a chimeric receptor approach (Isogaya et al., 1999; Kaumann and Molenaar, 2008), amino acids in the β1-adrenoceptor were mutated to those of the β2-adrenoceptor, and the pharmacological consequences observed. Here, we locate TM4, and specifically L195 and W199 within the human β1-adrenoceptor, as the major site responsible for the secondary conformation pharmacology.
Materials and Methods
Molecular biology reagents were from Promega (Madison, WI). Lipofectamine, OPTIMEM, pcDNA3.1, and Top 10F–competent cells were from Life Technologies (Paisley, UK). The QuikChange mutagenesis kit was from Stratagene (La Jolla, CA), and fetal calf serum was from PAA Laboratories (Teddington, Middlesex, UK). 3H-Adenine and 14C-cAMP were from Amersham International (Buckinghamshire, UK), and Ultima Gold XR scintillation fluid from PerkinElmer (Shelton, CT). Cimaterol, CGP 20712A, bisoprolol, and carvedilol were from Tocris Life Sciences (Avonmouth, UK). All other reagents were from Sigma-Aldrich (Poole, Dorset, UK). Racemic ligands were used throughout.
Molecular Biology
The cDNA sequence encoding the wild-type human β1-adrenoceptor (β1-WT) in pJG3.6 was a gift from Steve Rees (GlaxoSmithKline, Stevenage). This cDNA was subcloned as a HindIII/XbaI fragment into pcDNA3.1, and the sequence was confirmed by DNA sequencing. Mutations described in Table 1 were generated using QuikChange mutagenesis and BioLine PolyMate Additive for GC-rich templates (Baker et al., 2008). After subcloning in Top 10F–competent cells, each mutant β1-adrenoceptor cDNA was excised on HindIII/XbaI and subcloned into native pcDNA3.1 containing a neomycin selection marker. All mutations and sequences were confirmed by DNA sequencing using the School of Biomedical Sciences’ Sequencing Facility. To detect the most important areas of the human wild-type β1-adrenoceptor involved in the secondary conformation, we made point mutations in the transmembrane regions of the receptor such that the each TM region resembled that of the wild-type human β2-adrenoceptor (β2-WT). Prediction of the transmembrane regions was performed using ExPASy topology prediction tools (www.expasy.org). For example, six point mutations were made in β1-WT (L63I, L64V, A66S, L71A, A74F, V81T) which effectively converted the TM1 region of this receptor to that of the β2-WT. This chimeric receptor was called β1-TM1 (i.e., β1-adrenoceptor but with TM1 of the β2-WT, Table 1). This was then replicated for each TM region such that we had eight β1 receptor constructs; the β1-WT and one with each of the seven TM regions in turn mutated to that of the β2-adrenoceptor (Table 1). A similar set of mutations was made starting with the β2-WT and creating seven chimeric receptors, each with a TM region of the human β1-WT (Table 1). These constructs were expressed in Chinese hamster ovary (CHO) cells stably expressing a reporter construct with six cAMP response elements upstream of a secreted placental alkaline phosphatase reporter gene (CRE-SPAP) cells and in stable cell lines we generated (see below).
TABLE 1.
The chimeric β1/β2-adrenoceptor constructs, the amino acid changes made, and where a stable cell line was made; receptor expression levels are given as fmol/mg protein
| Constructs | Amino Acid Changes | Protein |
|---|---|---|
| fmol/mg | ||
| β1-WT | 556 ± 39 | |
| β1-TM1 | L63I, L64V, A66S, L71A, A74F, V81T | 372 ± 47 |
| β1-TM2 | M98T, S102C, L110A, T117A, I118H, V119I, V120L | 567 ± 58 |
| β1-TM3 | L133F, V137I, L154V | 1994 ± 214 |
| β1-TM4 | G177V, L178I, V179I, C180L, T181M, A184I, I185V, A187G, V189T, L195Q, W199Y | 233 ± 18 |
| β1-TM5 | R222Q, V230I, C238V, A241V, L245S | 359 ± 30 |
| β1-TM6 | V332T, L342I, A343V, V345I, K347H, A348V, F349I | 1207 ± 100 |
| β1-TM7 | R357E, L358V, F359Y, V360I, F361L, F362L, L365I, A368V, A371G, I375L | 2078 ± 133 |
| β1-A187G | A187G | |
| β1-V189T | V189T | |
| β1-L195Q | L195Q | |
| β1-W199Y | W199Y | |
| β1-TM4 stage 1 | G177V, L178I, V179I | |
| β1-TM4 stage 2 | G177V, L178I, V179I, C180L, T181M, A184I, I185V | |
| β1-TM4 stage 3 | G177V, L178I, V179I, C180L, T181M, A184I, I185V, A187G | |
| β1-TM4 stage 4 | G177V, L178I, V179I, C180L, T181M, A184I, I185V, A187G, V189T | |
| β1-TM4 stage 5 | G177V, L178I, V179I, C180L, T181M, A184I, I185V, A187G, V189T, L195Q | |
| β1-V189T-L195Q | V189T, L195Q | |
| β1-V189T-W199Y | V189T, W199Y | |
| β1-L195Q-W199Y | L195Q, W199Y | |
| β1-V189T-L195Q-W199Y | V189T, L195Q, W199Y | |
| β1-W199A | W199A | |
| β1-W199D | W199D | |
| β1-W199F | W199F | |
| β1-W199K | W199K | |
| β1-W199L | W199L | |
| β1-W199N | W199N | |
| β2-WT | 268 ± 25 | |
| β2-TM1 | I38L, V39L, S41A, A46L, F49A, T56V | 138 ± 8 |
| β2-TM2 | T73M, C77S, A85L, A92T, H93I, I94V, L95V | 405 ± 41 |
| β2-TM3 | F108L, I112V, V129L | 1037 ± 113 |
| β2-TM4a | V152G, I153L, I154V, L155C, M156T, I159A, V160I, G162A, T164V, Q170L | 458 ± 64 |
| β2-TM5 | Q197R, I205V, V213C, V216A, S220L | 849 ± 83 |
| β2-TM6 | T281V, I291L, V292A, I294V, H296K, V297A, I298F | 728 ± 58 |
| β2-TM7 | E306R, V307L, Y308F, I309V, L310F, L311F, I314L, V317A, G320A, L324I | 67 ± 5 |
Making the full change here (i.e., with Y174W) rendered the receptor nonfunctional (no binding and no functional responses). This chimera with 10 of the 11 amino acid substitutions was therefore used as the β2-TM4 mutant.
Once important TM regions for the secondary site were identified, to determine which individual amino acids were involved, several single point mutations and chimeric receptors were made (Table 1) and these constructs expressed in CHO-CRE-SPAP cells, either as stable mixed populations of cells (CRE-SPAP assays) or in transiently transfected cells (3H-cAMP accumulation assays).
Cell Culture.
All CHO cells were grown in Dulbecco’s modified Eagle’s medium nutrient mix F12 containing 10% fetal calf serum and 2 mM L-glutamine in a 37°C humidified 5% CO2 with 95% air atmosphere.
Generation of Stable Cell Lines.
CHO cells stably expressing a CRE-SPAP reporter gene were secondarily transfected with the wild-type human β1- or β2-adrenoceptor, or one of the full TM chimeric receptors (total 16 cell lines) using lipofectamine and OPTIMEM and selected for 3 weeks using resistance to geneticin (1 mg/ml for the receptor) and hygromycin (200 µg/ml for the CRE-SPAP reporter). Single clones were identified by dilution cloning and expanded to generate stable cell lines. These cell lines were used to identify the TM regions important for the secondary site. Receptor expression level in these stable cell lines was measured as previously described (Baker 2005b).
Generation of Stable Mixed Populations of Cells.
Here, the same parent CHO-CRE-SPAP cells were transfected with the wild-type human β1- or β2-adrenoceptor or a single point mutation or chimeric receptor and selected for 3 weeks using resistance to geneticin (1 mg/ml for the receptor) and hygromycin (200 µg/ml for the CRE-SPAP reporter). During this time the cells were passaged twice. The cells were then plated into 96-well plates for CRE-SPAP production experiments.
Generation of Transient Populations.
For transiently transfected cells used in the 3H-cAMP assays, the parent CHO-CRE-SPAP cells were transfected, as above, on day 1, the transfection reagents removed and replaced with media on day 2, the cells plated into 24-well plates on day 3, and the 3H-cAMP accumulation assay performed on day 4.
CRE-SPAP Production
Cells were grown to confluence in sterile, clear plastic, tissue culture–treated 96-well plates. The cells were then serum starved by removal of the media and addition of 100 µl serum-free media (sfm) per well for 24 hours before experimentation. At the start of each experiment, the sfm was removed from each well and 100 µl sfm or 100 µl sfm containing an antagonist at the final required concentration was added to each well and the cells incubated for 30 minutes at 37°C. Agonist in 10 μl sfm was then added to each well and the plates incubated for 5 hours. After 5 hours, all drugs and media were removed from each well and 40 µl sfm added to each well. The plates were then incubated for 1 hour at 37°C before being transferred to an oven preheated to 65°C and incubated for 30 minutes to destroy endogenous phosphatases. SPAP production was then measured by the addition of 100 µl 5 mM p-nitrophenyl phosphate per well (in diethanolamine buffer) and read on a Dynatech MRX plate reader at 405 nM.
3H-cAMP Accumulation
Cells were grown to confluence in sterile, clear plastic, tissue culture–treated 24-well plates. Cells were prelabeled with 3H-adenine by incubation for at least 2 hours with 2 μCi/ml 3H-adenine in media (0.5 ml per well). The cells were washed, then 1 ml sfm containing 1 mM 3-isobutyl-1-methylxanthine was added to each well and the cells incubated for 15 minutes at 37°C. Agonists (in 10 μl sfm) were added to each well and the plates incubated for 5 hours to maximize the responses (without altering the EC50 values or % maximum isoprenaline response observed (Baker, 2010a). The assay was terminated by adding 50 μl concentrated HCl per well, the plates frozen, thawed, and 3H-cAMP separated from other 3H-nucleotides by sequential Dowex and alumina column chromatography (using 14C-cAMP to determine column efficiency). Isoprenaline (10 µM) was used to define the maximal response in each plate of each experiment.
Data Analysis
Agonist responses were best described by a one-site sigmoidal concentration response curve using the following equation:
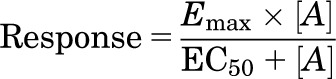 |
where Emax is the maximum response, [A] is the agonist concentration, and EC50 is the concentration of agonist that produces 50% of the maximal response.
The affinities of antagonists (KB values) were calculated from the rightward shift of the agonist concentration response curve in the presence of a fixed concentration of antagonist using the following:
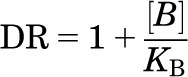 |
where DR (dose ratio) is the ratio of the agonist concentration required to stimulate an identical response in the presence and absence of a fixed concentration of antagonist [B].
When CGP12177 was used to antagonize the more efficacious agonists, clear partial agonism was seen (e.g., Fig. 2). Here, the affinity was initially calculated by the method of Stephenson (1956):
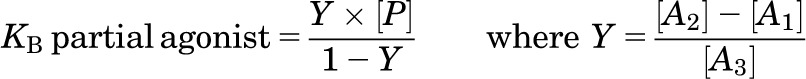 |
where [P] is the concentration of CGP12177, [A1] is the concentration of the agonist at the point where CGP12177 alone causes the same agonist response, [A2] is the concentration of agonist causing a given response above that achieved by CGP12177, and [A3] is the concentration of the agonist, in the presence CGP12177, causing the same stimulation as [A2].
Fig. 2.
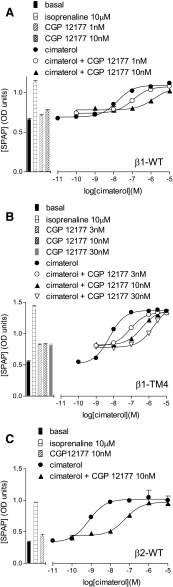
CRE-SPAP production in response to cimaterol in the absence and presence of CGP12177 in stable cell lines expressing (A) β1-WT, (B) β1-TM4, and (C) β2-WT. Bars represent basal CRE-SPAP production, that in response to 10 µM isoprenaline or that in response to 1–30 nM CGP12177 alone. Data points are mean ± S.E.M. of triplicate determinations. These single experiments are representative of (A) six, (B) eight, and (C) nine separate experiments. This figure shows that CGP12177 inhibits cimaterol responses with similar high affinity at β1-WT, β1-TM4, and β2-WT.
The response to pindolol (and at times CGP12177) was best described by a two-site concentration response (e.g., Fig. 6B)
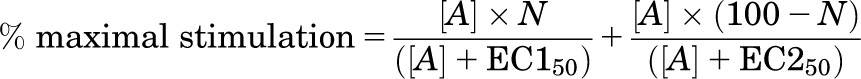 |
where N is the percentage of site 1, [A] is the concentration of agonist, and EC150 and EC250 are the respective EC50 values for the two agonist sites.
Fig. 6.
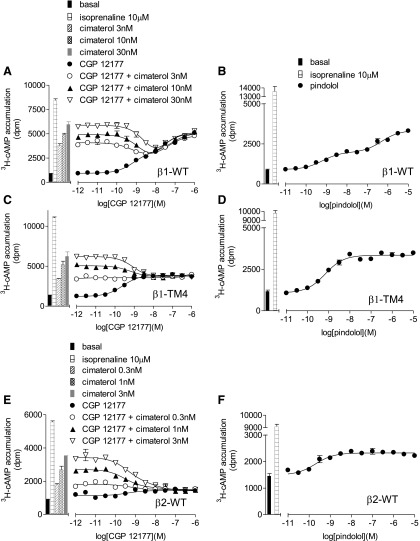
3H-cAMP accumulation in response to CGP12177 (A, C, and E) and pindolol (B, D, and F) in transiently transfected cells expressing β1-WT (A and B), β1-TM4 (C and D), and β2-WT (E and F). The CGP12177 responses were examined in the absence and presence of 3, 10, and 30 nM cimaterol (A and C); and 0.3, 1, and 3 nM cimaterol (E). Bars represent basal 3H-cAMP accumulation, that in response to 10 µM isoprenaline or that in response to 0.3–30 nM cimaterol alone. Data points are mean ± S.E.M. of triplicate determinations. These single experiments are representative of (A) six, (B) eight, (C) four, (D) three, (E) four, and (F) four separate experiments. This figure examines different evidence for the absence or presence of the secondary conformation. At β1-WT, low concentrations of CGP12177 (e.g., 3 nM, −8.5) inhibit the response to cimaterol, whereas higher concentrations of CGP12177 cause a stimulatory response (e.g., 100 nM, −7 and above). This creates a “dip” in the curve (CGP12177 + fixed concentration of cimaterol) and is strongly suggestive of interaction at two different conformations. This is absent at β1-TM4 and β2-WT, suggesting single-conformation interaction. Similarly, the response to pindolol is biphasic at β1-WT but monophasic at β1-TM4 and β2-WT, again suggesting two conformations at β1-WT but only one conformation at β1-TM4 and β2-WT.
A two-site analysis was also used for the experiments e.g., Fig. 6A:
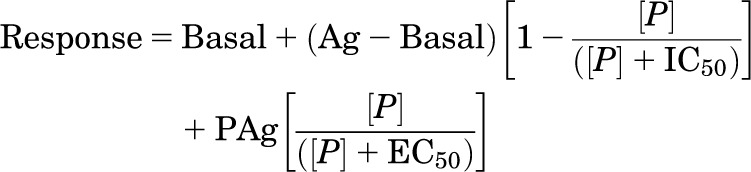 |
where basal is the response in the absence of agonist, Ag is the response to a fixed concentration of agonist, [P] is the concentration of partial agonist (e.g., CGP12177), IC50 is the concentration of competing partial agonist that inhibits 50% of the response of the fixed agonist, PAg is the maximum stimulation by the competing partial agonist, and EC50 is the concentration of competing agonist that stimulated a half maximal competing partial agonist response.
A 10 μM (maximal) isoprenaline concentration was included in each plate for each separate experiment for CRE-SPAP production and 3H-cAMP accumulation, to allow agonist responses to be expressed as a percentage of the isoprenaline maximum for each experiment. Data points in the figures are presented as mean ± S.E.M. of triplicate determinations from a single experiment. Data in the text and tables are mean ± S.E.M. of n separate experiments.
Results
Demonstration of the Two Agonist Conformations of the Human β1-Adrenoceptor: CRE-SPAP Production in Stable Cell Lines.
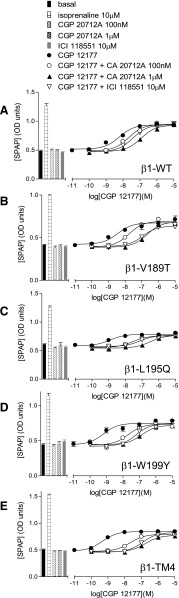
Cimaterol, a primary catecholamine conformation agonist (Baker 2005a), stimulated a response in cells stably expressing the human wild-type β1-adrenoceptor (log EC50 = −8.25 ± 0.14) that was 95.8 ± 2.9% that of the response to 10-µM isoprenaline (top half Table 2; Fig. 1A). As expected, this β1 response was readily inhibited by the β1-selective antagonist CGP20712A [2-hydroxy-5-(2-[{hydroxy-3-(4-[1-methyl-4-trifluoromethyl-2-imidazolyl]phenoxy)propyl}amino]ethoxy)benzamide] (log KB −9.18) and poorly inhibited by the β2-selective antagonist ICI118551 [(−)-1-(2,3-[dihydro-7-methyl-1H-inden-4-yl]oxy)-3-([1-methylethyl]-amino)-2-butanol] (log KB −6.96). CGP12177, a partial agonist, also inhibited this cimaterol response with high affinity indicating high affinity for the catecholamine conformation of the receptor (log KB −9.55; Fig. 2A; Table 2). CGP12177 is however also known to activate the secondary conformation of the β1-adrenoceptor. At the β1-WT, CGP12177 stimulated a partial agonist response, log EC50 −8.18 ± 0.08, which was 73.8 ± 5.6% that of the maximum to isoprenaline (Fig. 1B). This CGP12177 response was also inhibited by antagonists but in each case higher concentrations of antagonist were required, yielding lower log KB values (e.g., log KB for CGP20712A was −7.16; bottom half Table 2). The fact that 10–500-fold higher concentrations of antagonist were required to inhibit the CGP12177 rather than the cimaterol responses, and the discrepancy between the log KB value and the log EC50 value for CGP12177 itself, demonstrate the high affinity and the lower affinity conformations of the human β1-adrenoceptor.
TABLE 2.
Log EC50 values and % maximum response to isoprenaline for CRE-SPAP production for the agonists cimaterol and CGP12177 at the human β1-WT and chimeric β1/β2-adrenoceptors
Mutations in the β1-WT mean that each TM region in turn is mutated to that of the β2-WT (see Table 1). Log KB values for several antagonists for inhibition of the cimaterol and CGP12177 responses were determined. These data were obtained from stable cell lines, and n in the table refers to the number of separate experiments. The table shows that the most consistent and highly significant difference between the β1-WT receptor and all of the TM-swap mutants is that obtained from β1-TM4 when CGP12177 is the agonist. As CGP12177 agonist responses occur at the secondary low-affinity conformation of the β1-WT receptor, this suggests that the β1-TM4 receptor mutations alter this conformation in some way.
| Cimaterol Log EC50 | % Isoprenaline | n | Log KB CGP20712A | n | Log KB Bisoprolol | n | Log KB ICI118551 | n | Log KB Propranolol | n | Log KB Carvedilol | n | Log KB CGP12177 | n | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cimaterol as agonist: β1 stable cell lines | |||||||||||||||
| β1-WT | −8.25 ± 0.14 | 95.8 ± 2.9 | 8 | −9.18 ± 0.07 | 4 | −8.48 ± 0.17 | 5 | −6.96 ± 0.04 | 5 | −8.52 ± 0.08 | 12 | −9.89 ± 0.13 | 5 | −9.55 ± 0.12 | 8 |
| β1-TM1 | −7.91 ± 0.05* | 96.5 ± 3.3 | 11 | −9.35 ± 0.10 | 9 | −8.65 ± 0.12 | 5 | −6.96 ± 0.05 | 9 | −8.66 ± 0.07 | 16 | −9.88 ± 0.08 | 5 | −9.77 ± 0.07 | 15 |
| β1-TM2 | −8.19 ± 0.03 | 98.9 ± 3.0 | 15 | −8.78 ± 0.12 | 8 | −8.15 ± 0.07 | 7 | −7.47 ± 0.03* | 14 | −8.52 ± 0.03 | 24 | −9.53 ± 0.18 | 8 | −9.90 ± 0.09 | 17 |
| β1-TM3 | −8.82 ± 0.03* | 102.4 ± 2.5 | 9 | −9.34 ± 0.12 | 9 | −8.43 ± 0.07 | 4 | −7.31 ± 0.04* | 10 | −8.53 ± 0.06 | 15 | −9.92 ± 0.16 | 5 | −9.49 ± 0.06 | 13 |
| β1-TM4 | −8.15 ± 0.02 | 99.2 ± 1.8 | 10 | −9.50 ± 0.13 | 11 | −8.81 ± 0.04 | 5 | −8.07 ± 0.06* | 9 | −8.99 ± 0.04* | 15 | −9.81 ± 0.12 | 6 | −9.63 ± 0.08 | 17 |
| β1-TM5 | −8.48 ± 0.05 | 103.0 ± 1.7 | 9 | −9.09 ± 0.04 | 11 | −7.91 ± 0.03 | 5 | −7.01 ± 0.06 | 11 | −8.71 ± 0.04 | 17 | −9.96 ± 0.09 | 6 | −9.68 ± 0.09 | 17 |
| β1-TM6 | −8.59 ± 0.03* | 103.2 ± 2.2 | 10 | −8.52 ± 0.07 | 8 | −8.10 ± 0.15 | 5 | −7.92 ± 0.05* | 12 | −8.90 ± 0.05* | 18 | −10.13 ± 0.09 | 6 | −9.58 ± 0.06 | 14 |
| β1-TM7 | −8.54 ± 0.05 | 106.1 ± 2.5 | 10 | −8.45 ± 0.08 | 7 | −8.19 ± 0.06 | 5 | −7.80 ± 0.05* | 12 | −8.95 ± 0.05* | 18 | −10.06 ± 0.03 | 3 | −9.39 ± 0.05 | 13 |
| CGP12177 Log EC50 | % Isoprenaline | n | Log KB CGP20712A | n | Log KB Bisoprolol | n | Log KB ICI118551 | n | Log KB Propranolol | n | Log KB Carvedilol | n | |||
| CGP12177 as agonist: β1 stable cell lines | |||||||||||||||
| β1-WT | −8.18 ± 0.08 | 73.8 ± 5.6 | 10 | −7.16 ± 0.06 | 14 | −5.83 ± 0.14 | 4 | −5.90 ± 0.15 | 9 | −6.18 ± 0.06 | 13 | −7.25 ± 0.18 | 7 | ||
| β1-TM1 | −7.83 ± 0.05 | 53.7 ± 3.9 | 10 | −7.41 ± 0.13 | 11 | −5.77 ± 0.18 | 4 | −5.79 ± 0.09 | 3 | −6.55 ± 0.12 | 13 | −7.72 ± 0.13 | 7 | ||
| β1-TM2 | −8.27 ± 0.09 | 53.5 ± 3.9 | 14 | −7.13 ± 0.08 | 13 | −6.72 ± 0.18 | 7 | −5.87 ± 0.05 | 6 | −6.87 ± 0.15* | 18 | −8.52 ± 0.15*,** | 7 | ||
| β1-TM3 | −8.07 ± 0.03 | 70.5 ± 3.6 | 9 | −7.45 ± 0.05 | 12 | −5.83 ± 0.13 | 4 | −5.83 ± 0.09 | 7 | −6.31 ± 0.05 | 16 | −7.45 ± 0.09 | 6 | ||
| β1-TM4 | −9.27 ± 0.06*,** | 29.2 ± 1.4*,** | 10 | −9.49 ± 0.08*,** | 9 | −8.83 ± 0.21*,** | 7 | −7.93 ± 0.11*,** | 11 | −8.85 ± 0.09*,** | 15 | −9.73 ± 0.21*,** | 7 | ||
| β1-TM5 | −7.91 ± 0.03 | 60.1 ± 3.4 | 10 | −6.68 ± 0.04* | 11 | −5.31 ± 0.13 | 5 | −5.71 ± 0.15 | 8 | −6.23 ± 0.09 | 15 | −7.53 ± 0.16 | 7 | ||
| β1-TM6 | −7.83 ± 0.02* | 63.5 ± 2.8 | 11 | −5.97 ± 0.08*,** | 12 | −5.22 ± 0.14 | 3 | −5.79 ± 0.04 | 11 | −6.05 ± 0.06 | 19 | −7.24 ± 0.18 | 7 | ||
| β1-TM7 | −7.08 ± 0.02*,** | 68.5 ± 3.8 | 11 | −6.86 ± 0.05 | 10 | −5.67 ± 0.05 | 5 | −6.67 ± 0.03*,** | 13 | −6.42 ± 0.06 | 16 | −7.35 ± 0.12 | 6 | ||
P < 0.001; one-way ANOVA with post hoc Newman–Keuls comparing values from the mutant receptors with those obtained from the β1-WT. Thus, the log EC50 for CGP12177 at β1-TM4 is different from that obtained from the β1-WT with P < 0.001; **P < 0.001; one-way ANOVA with post hoc Newman–Keuls comparing each value with all other values in this set. Thus, the log EC50 value for CGP12177 at β1-TM4 is different from those obtained for β1-WT, β1-TM1, β1-TM2, β1-TM3, β1-TM5, β1-TM6, and β1-TM7, with P < 0.001 in all cases.
Fig. 1.
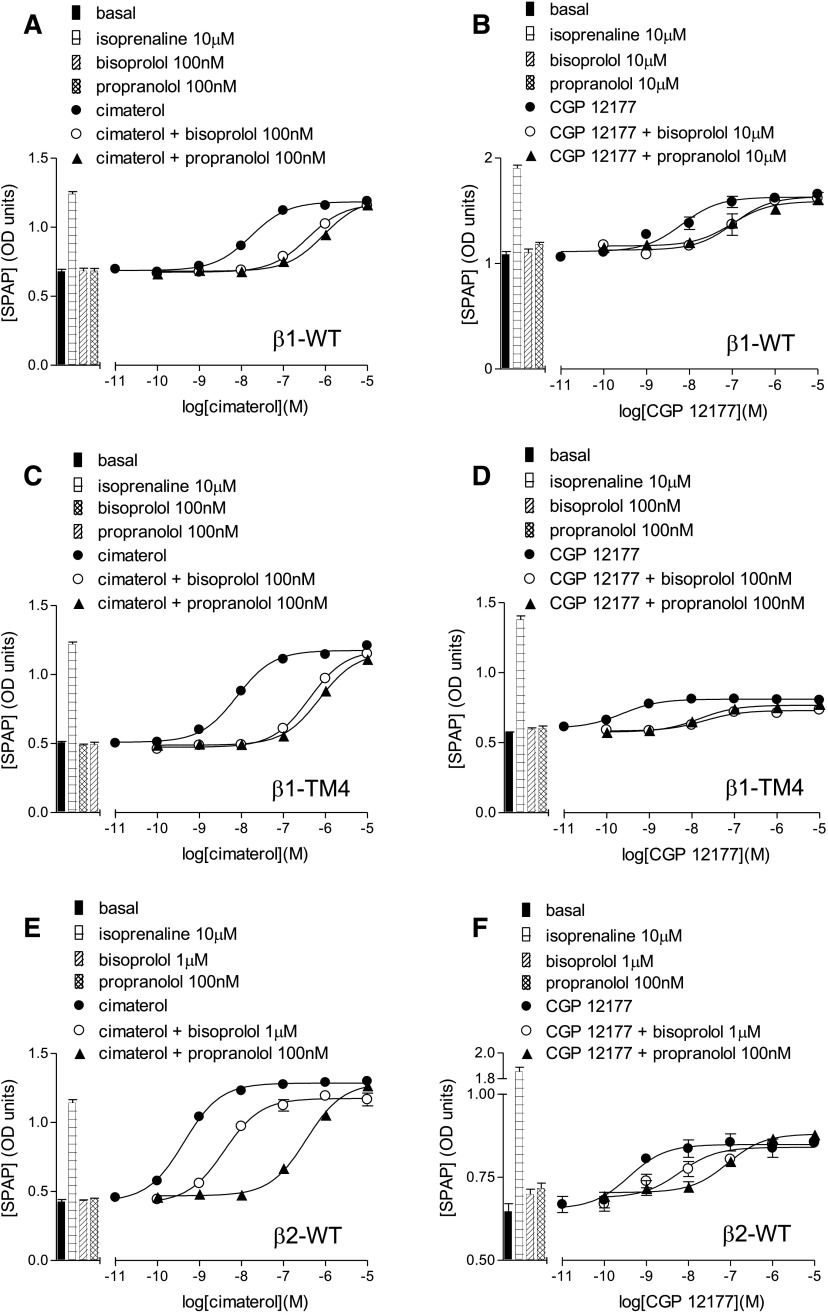
CRE-SPAP production in response to cimaterol (A, C, and E) and CGP12177 (B, D, and F) in the absence and presence of bisoprolol and propranolol in stable cell lines expressing β1-WT (A and B), β1-TM4 (C and D), and human β2-WT (E and F). Bars represent basal CRE-SPAP production, that in response to 10 µM isoprenaline or that in response to bisoprolol or propranolol alone. Data points are mean ± S.E.M. of triplicate determinations. These single experiments are representative of (A) five, (B) four, (C) four, (D) seven, (E) six, and (F) 10 separate experiments. This figure shows that the greater concentrations of antagonist are required to inhibit the secondary conformation CGP12177 responses in the β1-WT, but that this is not the case for the β1-TM4 or β2-WT.
At the human β2-adrenoceptor, agonist responses to cimaterol and CGP12177 were also observed; however, similar concentrations of antagonist were required to inhibit these responses, yielding similar log KB values (Fig. 1, E and F; Table 4), and the log KB and log EC50 values for CGP12177 were similar, suggesting that cimaterol and CGP12177 are acting through the same conformation of the β2-adrenoceptor.
TABLE 4.
Log EC50 values and % maximum response to isoprenaline for CRE-SPAP production for the agonists cimaterol and CGP12177 at the human β2-WT and chimeric β2/β1-receptors, where mutations in the β2-WT mean that each TM region in turn is mutated to that of the β1-WT (see Table 1)
Log KB values for several antagonists for inhibition of the cimaterol and CGP12177 responses are also given. These data were obtained from stable cell lines; n refers to the number of separate experiments. Although there are some differences among the mutant receptors (e.g., β2-TM2 has higher affinity for CGP20712A than β2-WT), there is no highly significant consistent change in one mutant receptor compared with the others.
| Cimaterol Log EC50 |
% Isoprenaline |
n |
Log KB CGP20712A |
n |
Log KB Bisoprolol |
n |
Log KB ICI118551 |
n |
Log KB Propranolol |
n |
Log KB CGP12177 |
n |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cimaterol as agonist: β2 stable cell lines | |||||||||||||
| β2-WT | −9.05 ± 0.08 | 101.5 ± 3.2 | 13 | −6.03 ± 0.12 | 8 | −6.85 ± 0.10 | 8 | −9.56 ± 0.07 | 8 | −9.65 ± 0.05 | 14 | −10.00 ± 0.05 | 9 |
| β2-TM1 | −9.45 ± 0.06 | 105.3 ± 2.8 | 9 | −6.46 ± 0.10 | 8 | −6.82 ± 0.09 | 10 | −9.74 ± 0.08 | 9 | −9.56 ± 0.06 | 19 | −9.74 ± 0.11 | 19 |
| β2-TM2 | −9.64 ± 0.08* | 102.1 ± 1.8 | 12 | −7.20 ± 0.11* | 8 | −7.25 ± 0.10 | 7 | −9.30 ± 0.11 | 8 | −9.87 ± 0.03 | 14 | −10.01 ± 0.06 | 9 |
| β2-TM3 | −9.21 ± 0.13 | 98.3 ± 4.1 | 7 | −6.57 ± 0.12 | 7 | −7.05 ± 0.13 | 6 | −9.71 ± 0.10 | 7 | −9.81 ± 0.07 | 11 | −10.00 ± 0.10 | 10 |
| β2-TM4 | −9.66 ± 0.06* | 103.3 ± 2.4 | 13 | −6.09 ± 0.09 | 10 | −6.75 ± 0.03 | 9 | −8.87 ± 0.04* | 10 | −9.34 ± 0.06 | 14 | −9.83 ± 0.05 | 9 |
| β2-TM5 | −8.97 ± 0.09 | 100.6 ± 2.4 | 13 | −6.63 ± 0.12 | 10 | −7.04 ± 0.09 | 9 | −9.50 ± 0.09 | 9 | −9.46 ± 0.04 | 13 | −9.82 ± 0.07 | 4 |
| β2-TM6 | −8.74 ± 0.05 | 101.0 ± 4.7 | 12 | −7.05 ± 0.15* | 7 | −7.07 ± 0.10 | 9 | −8.86 ± 0.12* | 9 | −9.52 ± 0.06 | 11 | −9.59 ± 0.13 | 8 |
| β2-TM7 | −8.77 ± 0.06 | 107.4 ± 2.9 | 10 | −6.54 ± 0.04 | 14 | −7.06 ± 0.09 | 11 | −9.18 ± 0.07 | 12 | −9.30 ± 0.06* | 17 | −9.72 ± 0.10 | 13 |
| CGP12177 Log EC50 | % Isoprenaline | n | Log KB CGP20712A | n | Log KB Bisoprolol | n | Log KB ICI118551 | n | Log KB Propranolol | n | |||
| CGP12177 as agonist: β2 stable cell lines | |||||||||||||
| β2-WT | −9.35 ± 0.09 | 23.5 ± 3.9 | 14 | −6.12 ± 0.09 | 3 | −6.60 ± 0.14 | 11 | −9.31 ± 0.17 | 11 | −9.02 ± 0.12 | 19 | ||
| β2-TM1 | −9.48 ± 0.19 | 26.9 ± 1.7 | 9 | −6.00 ± 0.16 | 9 | −6.63 ± 0.12 | 12 | −9.51 ± 0.17 | 12 | −9.07 ± 0.08 | 20 | ||
| β2-TM2 | −9.20 ± 0.09 | 12.6 ± 1.9 | 11 | −6.79 ± 0.11 | 8 | −7.00 ± 0.09 | 9 | −9.03 ± 0.11 | 10 | −9.32 ± 0.10 | 14 | ||
| β2-TM3 | −9.19 ± 0.10 | 35.9 ± 3.3 | 9 | −6.21 ± 0.29 | 4 | −6.53 ± 0.12 | 8 | −9.47 ± 0.16 | 8 | −8.95 ± 0.09 | 21 | ||
| β2-TM4 | −9.20 ± 0.07 | 38.5 ± 2.4* | 13 | −6.05 ± 0.12 | 8 | −6.34 ± 0.09 | 7 | −8.42 ± 0.08* | 11 | −8.34 ± 0.08* | 18 | ||
| β2-TM5 | −9.31 ± 0.07 | 28.8 ± 2.0 | 12 | −6.47 ± 0.15 | 8 | −6.98 ± 0.22 | 8 | −9.31 ± 0.08 | 9 | −8.75 ± 0.10 | 19 | ||
| β2-TM6 | −8.81 ± 0.10 | 22.4 ± 1.6 | 8 | −7.31 ± 0.34 | 5 | −6.74 ± 0.11 | 5 | −8.39 ± 0.27 | 4 | −9.11 ± 0.11 | 4 | ||
| β2-TM7 | −9.71 ± 0.12 | 41.3 ± 2.7* | 9 | −6.19 ± 0.15 | 10 | −6.59 ± 0.16 | 10 | −9.09 ± 0.14 | 9 | −9.02 ± 0.08 | 22 | ||
P < 0.001 One-way ANOVA with post hoc Newman–Keuls comparing values from the mutant receptors with those obtained from the β2-WT. Thus, the log KB value for CGP20712A at β2-TM2 obtained in the presence of cimaterol is different (P < 0.001) from that obtained in the β2-WT receptor. In contrast to data on the β1-receptor in Table 2, there are no values with P < 0.001 for one-way analysis of variance with post hoc Newman–Keuls comparing each value with all other values in this set.
Identification of Transmembrane Regions Important in the Secondary Conformation of the β1-Adrenoceptor: CRE-SPAP Production in Stable Cell Lines.
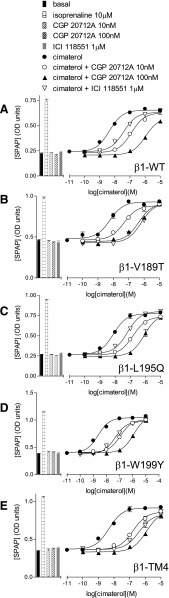
To identify areas in the receptor important for the secondary conformation, β1/β2 chimeric receptors were examined. These receptors had mutations such that each TM was in turn swapped for that of the other receptor (e.g., β1-TM1 is the β1-adrenoceptor sequence containing mutations that make it equivalent to the TM1 region of the β2-adrenoceptor) (Table 1). When the β1-TM chimeric receptors were examined (each stably expressed in a different cell line), cimaterol stimulated a full agonist response at all β1/β2 receptors (β1-TM1 through to β1-TM7) (Fig. 1C; top half Table 2) and this response was readily inhibited by CGP20712A and poorly by ICI118551, confirming that despite changing each transmembrane in turn to that of the β2-adrenoceptor, these chimeric receptors retained a β1-selective nature. There were some differences in the affinity of antagonists (e.g., affinity of ICI118551 at the β1-TM2, β1-TM3, β1-TM4, β1-TM6, and β1-TM7 receptors is substantially higher than at the β1-WT; top half Table 2). This could be due to a general trend of these chimeras “losing” β1-subtype selectivity as judged by a range of ligands or due to a specific interaction between the ligand ICI118551 and specific amino acids in these chimeras. To determine which, several antagonist ligands (bisoprolol a less selective but still a β1-selective ligand; propranolol and carvedilol slightly β2-selective ligands) were examined to generate a pattern of affinity (top half Table 2; Fig. 1). The affinity of CGP20712A and bisoprolol remained high (similar to β1-WT, top half Table 2 and very different from values obtained at the β2-WT; top half Table 4). Thus, the overall pattern for these chimeras is one of retaining β1-selective properties (and indeed ICI118551 was later found to interact with a specific amino acid in TM4 rather than a general pattern across several ligands; see below).
When the CGP12177 responses were examined, the data achieved for β1-TM4 were significantly different from those at the other chimeras (P < 0.001; bottom half Table 2; Fig. 1D). Firstly, the log EC50 for CGP12177 was very different from that at the other receptors (bottom half Table 2). Secondly, the log KB value and log EC50 values for CGP12177 in the β1-TM4 cells were similar (left column, Table 3, and indeed similar to that seen in the β2-WT cells; Table 4). Finally, the affinities of antagonists in the presence of CGP12177 at β1-TM4 were very similar to those obtained when cimaterol was used as the agonist in these cells (Table 2). This is most easily seen in Table 3, where the ratios of the antagonist affinities are compared. Whereas the ratio of antagonist affinities for CGP20712A at β1-WT was log 2.02 (e.g., it has 100-fold different affinity for the two conformations), it was log 0.01 at β1-TM4, suggesting that there is no difference in affinity and that the secondary conformation was not in existence. Thus, β1-TM4 had a pharmacological profile consistent with CGP12177 acting as a partial agonist at a single high-affinity conformation of the β1-adrenoceptor (i.e., in a similar manner to which CGP12177 acts at the single receptor conformation of the β2-WT adrenoceptor).
TABLE 3.
The ratio of the log KB value for CGP12177 (as an antagonist of cimaterol; thus, at the high-affinity conformation) and log EC50 value for CGP12177 (as an agonist of the secondary low-affinity conformation) for responses seen at the β1-WT and β1-TM chimeric receptors (data taken from Table 2)
The ratios of the affinity for antagonists at the two conformations (i.e., log KB for antagonism of cimaterol at high-affinity site/log KB for antagonism of CGP12177 at low-affinity site) were determined. This table examines the ratio of the affinities of ligands at the two conformations of the β1-adrenoceptor and thus allows for differences in absolute affinity. For example, ICI118551 has a higher affinity at the β1-TM7 receptor (Table 2) than the β1-WT receptor; however, the ratio of the affinity in the presence of cimaterol and CGP 12177 is similar to that at the WT receptor, suggesting that although the β1-TM7 mutation alters the affinity of ICI118551, it does not alter the secondary conformation. The most consistent change is seen with the β1-TM4 mutation, where the ratios are all nearly 0, suggesting no difference in affinity (i.e., no secondary conformation).
| Ratio of Log KB CGP12177/ Log EC50 CGP12177 |
Ratio of Log KB (Cimaterol as Agonist)/Log KB (CGP12177 as Agonist) | |||||
|---|---|---|---|---|---|---|
| CGP20712A |
Bisoprolol |
ICI118551 |
Propranolol |
Carvedilol |
||
| β1-WT | 1.37 | 2.02 | 2.65 | 1.06 | 2.34 | 2.64 |
| β1-TM1 | 1.94 | 1.94 | 2.88 | 1.17 | 2.11 | 2.16 |
| β1-TM2 | 1.63 | 1.65 | 1.43 | 1.60 | 1.65 | 1.01 |
| β1-TM3 | 1.42 | 1.89 | 2.60 | 1.48 | 2.22 | 2.47 |
| β1-TM4 | 0.36 | 0.01 | −0.02 | 0.14 | 0.14 | 0.08 |
| β1-TM5 | 1.77 | 2.41 | 2.60 | 1.30 | 2.47 | 2.43 |
| β1-TM6 | 1.75 | 2.55 | 2.88 | 2.15 | 2.85 | 2.89 |
| β1-TM7 | 2.31 | 1.59 | 2.52 | 1.13 | 2.53 | 2.71 |
When the full β2-TM4 construct was expressed (11 amino acid changes), no function or binding was detectable despite multiple attempts. Therefore, a construct containing 10 amino acid changes (all except the last Y174W) was made, which was then used as β2-TM4 (Tables 1, 4, and 5). The data obtained for the β2/β1 chimeras (Table 4) suggest that the agonist responses and antagonist affinities obtained change very little in the β2-adrenoceptors with whole TM region changes.
TABLE 5.
The ratio of the log KB value for CGP12177 (as an antagonist of cimaterol) and log EC50 value for CGP12177 for responses seen at the β2-WT and β2-TM chimeric receptors (data taken from Table 4)
Ratios of the affinity for antagonists at the two conformations are also given (i.e., log KB for antagonism of cimaterol/log KB for antagonism of CGP12177). This table examines the ratio of the affinities of ligands at the β2-adrenoceptor and thus allows for differences in absolute affinity. ICI118551 has lower affinity at the β2-TM4 receptor (Table 4), but this occurred both in the presence of cimaterol and CGP12177; thus, the ratio is similar to that at the other receptors. There is no large consistent change with any of the receptors.
| Ratio of Log KB CGP12177/Log EC50 CGP12177 | Ratio of Log KB (Cimaterol as Agonist)/Log KB (CGP12177 as Agonist) |
||||
|---|---|---|---|---|---|
| CGP20712A | Bisoprolol | ICI118551 | Propranolol | ||
| β2-WT | 0.65 | −0.09 | 0.25 | 0.25 | 0.63 |
| β2-TM1 | 0.26 | 0.46 | 0.19 | 0.23 | 0.49 |
| β2-TM2 | 0.81 | 0.41 | 0.25 | 0.27 | 0.55 |
| β2-TM3 | 0.81 | 0.36 | 0.52 | 0.24 | 0.86 |
| β2-TM4 | 0.63 | 0.04 | 0.41 | 0.45 | 1.00 |
| β2-TM5 | 0.51 | 0.16 | 0.06 | 0.19 | 0.71 |
| β2-TM6 | 0.78 | −0.26 | 0.33 | 0.47 | 0.41 |
| β2-TM7 | 0.01 | 0.35 | 0.47 | 0.09 | 0.28 |
Examination of the ratios of antagonist affinities at the two conformations is again helpful for detecting the most important changes (Tables 3 and 5). Thus, although there are some differences for the chimeric receptors (e.g., EC50/KB ratio for β1-TM7 and β2-TM7), by far the most overwhelming differences are in the β1-TM4 chimeric receptor; this is clearly shown when comparing the ratios of the log KB and log EC50 values at the two conformations, as shown in Tables 3 and 5. Also, here, although ICI118551 was found to have higher affinity at β1-TM4 (and indeed β1-TM7), the ratio of the affinities for the two conformations of the β1-adrenoceptor clearly shows that β1-TM4 is different from the other β1-chimeric receptors.
Importantly, because the stable cell lines used here have different levels of receptor expression (Table 1), neither of these methods of assessing the presence of the secondary conformation relies on the receptor expression levels being the same. Antagonist affinity measurements are independent of receptor expression level and, because CGP12177 is a partial agonist in all of these cell lines, its KB value in a given cell line should be similar to its EC50 value in that cell line (i.e., ratio of zero) if the two are acting at a single conformation. A further piece of evidence can be obtained from comparing the log EC50 values for CGP12177 over the cell lines, although this is more problematic, not least as it is likely to depend on receptor expression levels. Interestingly here, the log EC50 for CGP12177 at β1-TM4 is markedly more potent (left-shifted) even though its receptor expression level is the lowest of the β1 cell lines and, thus, if anything, would be expected to have a less potent (i.e., right-shifted) log EC50 value. Even this softer evidence is therefore pointing to β1-TM4 as an important region for the secondary conformation.
Identification of the Amino Acids in TM4 Involved in the Secondary Conformation: CRE-SPAP Production in Stable Mixed Populations of Cells.
To determine which of the amino acids in TM4 were responsible for this secondary conformation, intermediate stages of the production of the chimeric receptors were examined that had several, but not all, of the amino acids changes required to alter the TM domain from β1 to β2 (see Table 1 for the amino acid changes made). These constructs were expressed in parent CHO-SPAP cells and experiments performed in stable mixed populations of cells (4–24 separate populations of cells were generated for each chimeric receptor). Whereas the affinity for antagonists remained very similar when cimaterol was the agonist (primary catecholamine site, top half of Table 6, with the exception of ICI118551), when the secondary site conformation was examined, (i.e., with CGP12177 as the agonist), the log EC50 value for CGP12177 became more potent (approaching that for the log KB value) in β1-TM4 stage 5 (bottom half Table 6). Secondly, the affinity of the other antagonists (log KB values) also became higher with β1-TM4 stage 5 and full β1-TM4 chimeras (bottom half Table 6). The affinity for ICI118551 was high at both conformations for β1-TM4 stage 4, although the ratio (Table 7) suggests the retention of both conformations of the receptor. This suggests that amino acids L195 and W199 are the most important for the secondary conformation.
TABLE 6.
Log EC50 values and % maximum response to isoprenaline for CRE-SPAP production for the agonists cimaterol and CGP12177 at the β1-WT, the chimeric β1-TM4 receptor (containing the full TM4 mutations), and chimeric β1/β2-adrenoceptors with several amino acids in TM4 mutated to that of the β2-WT
Log KB values for several antagonists for inhibition of the cimaterol and CGP12177 responses are also given. Table 1 lists the detail of each mutation. These data were obtained from between 4 and 24 stable mixed populations of cells for each chimera, and n in the table refers to the number of separate experiments. This table shows that within TM4, it is only the amino acid changes in stage 5 and the full TM4 (i.e., L195Q and W199Y) that are very important for the alteration of the secondary conformation (i.e., responses to CGP12177 and antagonist affinities obtained in the presence of CGP12177). Stage 4 (addition of V189T to the other changes) affects the affinity of ICI18551. There is no change in the pharmacology of either conformation, even when the majority of amino acids have been changed (up to and including stage 3).
| Cimaterol Log EC50 |
Isoprenaline
% |
n |
Log KB CGP20712A |
n |
Log KB ICI118551 |
n |
Log KB Propranolol |
n |
Log KB CGP12177 |
n |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cimaterol as agonist: stable mixed populations of cells | |||||||||||
| β1-WT | −8.29 ± 0.04 | 81.2 ± 2.1 | 24 | −9.36 ± 0.07 | 35 | −7.13 ± 0.06 | 31 | −8.69 ± 0.08 | 30 | −10.01 ± 0.06 | 33 |
| β1-TM4 stage 1 | −8.26 ± 0.11 | 85.6 ± 3.9 | 4 | −9.10 ± 0.09 | 4 | −7.04 ± 0.09 | 6 | −8.64 ± 0.14 | 4 | −9.96 ± 0.33 | 3 |
| β1-TM4 stage 2 | −8.20 ± 0.06 | 77.8 ± 1.5 | 12 | −9.34 ± 0.10 | 18 | −7.14 ± 0.06 | 14 | −8.79 ± 0.11 | 12 | −9.81 ± 0.09 | 14 |
| β1-TM4 stage 3 | −8.13 ± 0.07 | 78.2 ± 3.9 | 11 | −8.95 ± 0.09 | 13 | −7.00 ± 0.07 | 10 | −8.63 ± 0.07 | 11 | −9.93 ± 0.06 | 15 |
| β1-TM4 stage 4 | −8.08 ± 0.05 | 76.3 ± 1.5 | 12 | −9.14 ± 0.08 | 18 | −7.62 ± 0.05* | 16 | −8.59 ± 0.06 | 12 | −9.77 ± 0.06 | 19 |
| β1-TM4 stage 5 | −8.14 ± 0.06 | 78.2 ± 3.2 | 11 | −8.97 ± 0.09 | 16 | −7.71 ± 0.07* | 15 | −8.81 ± 0.04 | 11 | −9.93 ± 0.08 | 19 |
| β1-TM4 | −8.32 ± 0.05 | 77.3 ± 1.9 | 23 | −9.43 ± 0.09 | 35 | −7.94 ± 0.06* | 31 | −9.08 ± 0.07 | 29 | −9.98 ± 0.09 | 35 |
| CGP12177 Log EC50 | Isoprenaline % | n | Log KB CGP20712A | n | Log KB ICI118551 | n | Log KB Propranolol | n | |||
| CGP12177 as agonist: stable mixed populations of cells | |||||||||||
| β1-WT | −8.12 ± 0.07 | 53.3 ± 1.5 | 21 | −7.37 ± 0.08 | 37 | −5.86 ± 0.08 | 22 | −6.69 ± 0.08 | 27 | ||
| β1-TM4 stage 1 | −8.43 ± 0.06 | 50.4 ± 2.2 | 4 | −7.32 ± 0.25 | 5 | −6.21 ± 0.50 | 3 | −6.17 ± 0.17 | 4 | ||
| β1-TM4 stage 2 | −8.46 ± 0.07 | 46.5 ± 1.9 | 11 | −7.55 ± 0.12 | 17 | −6.08 ± 0.11 | 10 | −6.78 ± 0.09 | 20 | ||
| β1-TM4 stage 3 | −8.48 ± 0.08 | 45.9 ± 3.0 | 11 | −7.13 ± 0.10 | 18 | −5.90 ± 0.11 | 10 | −6.88 ± 0.11 | 13 | ||
| β1-TM4 stage 4 | −7.93 ± 0.12 | 40.0 ± 1.6* | 10 | −7.26 ± 0.10 | 15 | −6.57 ± 0.13* | 10 | −6.98 ± 0.13 | 18 | ||
| β1-TM4 stage 5 | −9.29 ± 0.06* | 30.2 ± 2.3* | 11 | −8.74 ± 0.09* | 19 | −7.54 ± 0.09* | 10 | −8.18 ± 0.08* | 25 | ||
| β1-TM4 | −9.43 ± 0.06* | 33.9 ± 1.5* | 19 | −8.98 ± 0.07* | 39 | −7.64 ± 0.08* | 32 | −8.39 ± 0.06* | 40 | ||
P < 0.001; one-way ANOVA with post hoc Newman-Keuls comparing values from the mutant receptors with those obtained from the β1-WT; thus, the log EC50 for CGP12177 at β1-TM4 Stage 5 receptor is different from that obtained from the β1-WT with P < 0.001
TABLE 7.
The ratio of the log KB value for CGP12177 (as an antagonist of cimaterol; thus, at the high affinity conformation) and log EC50 value for CGP12177 (as an agonist of the secondary low affinity conformation) for responses seen at the β1-WT and β1-TM4 chimeric receptors (data taken from Table 6)
Ratio of the affinity for antagonists at the two conformations is given (i.e., log KB for antagonism of cimaterol at high affinity site/log KB for antagonism of CGP12177 at low-affinity site). This table examines the ratio of the affinities of ligands and shows that the ratio of affinities between the two conformations is much less for β1-TM4 stage 5 and beyond, suggesting that only the amino acids in stage 5 and beyond are important for the secondary conformation.
| Ratio of Log KB CGP12177/Log EC50 CGP12177 | Ratio of Log KB (Cimaterol as Agonist)/Log KB (CGP12177 as Agonist) |
|||
|---|---|---|---|---|
| CGP20712A | ICI118551 | Propranolol | ||
| β1-WT | 1.89 | 1.99 | 1.27 | 2.00 |
| β1-TM4 stage 1 | 1.53 | 1.78 | 0.83 | 2.47 |
| β1-TM4 stage 2 | 1.35 | 1.79 | 1.06 | 2.01 |
| β1-TM4 stage 3 | 1.45 | 1.82 | 1.10 | 1.75 |
| β1-TM4 stage 4 | 1.84 | 1.88 | 1.05 | 1.61 |
| β1-TM4 stage 5 | 0.64 | 0.23 | 0.17 | 0.63 |
| β1-TM4 | 0.55 | 0.45 | 0.30 | 0.69 |
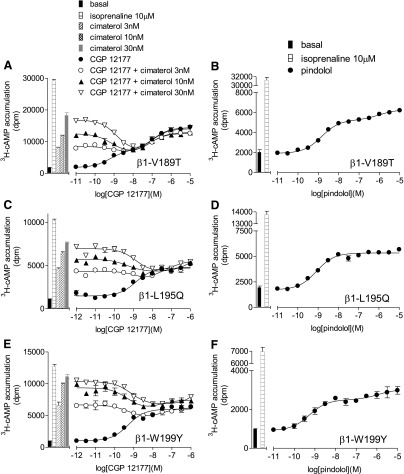
To investigate the role of these specific amino acids further, each of these individual single point mutations were examined alone (in stable mixed populations) rather than on the background of the other TM4 mutations (Tables 1, 8, and 9; Figs. 3 and 4). β1-L195Q and β1-W199Y had little effect on the cimaterol catecholamine conformation response (top half Table 8; Fig. 3). However, CGP12177 agonist responses were significantly more potent at β1-L195Q and β1-W199Y (bottom half Table 8; Fig. 4). Examining antagonist affinity values, whereas β1-V189T affected the affinity of ICI118551 at the catecholamine conformation, the affinities of CGP20712A, propranolol, and CGP12177 were largely unaffected by any of the mutations (top half of Table 8; Fig. 3). However, the secondary conformation CGP12177 responses were significantly more sensitive to inhibition with the L195Q and W199Y mutations (bottom half Table 8; Fig. 4).
TABLE 8.
Log EC50 values and % maximum response to isoprenaline for CRE-SPAP production for the agonists cimaterol and CGP12177 at β1-WT, the chimeric β1-TM4 receptor (containing the full TM4 mutations), and chimeric β1/β2-adrenoceptors with single point mutations and then combinations of these mutations
Log KB values for several antagonists for inhibition of the cimaterol and CGP12177 responses are given. These data were obtained from between 4 and 24 stable mixed populations of cells for each receptor, and n in the table refers to the number of separate experiments. This table shows that the mutations L195Q and W199Y, both alone and in combination, have a very significant effect on the responses to CGP12177 and affinity of antagonist measured in the presence of CGP12177 (i.e., the secondary conformation of the β1-receptor). Every time the mutation V189T is present, the affinity of ICI118551 is increased, both at the catecholamine conformation (in the presence of cimaterol) and at the secondary conformation (presence of CGP12177).
| Cimaterol Log EC50 | % Isoprenaline | n | Log KB CGP20712A | n | Log KB ICI118551 | n | Log KB propranolol | n | Log KB CGP12177 | n | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cimaterol as agonist: stable mixed populations of cells | |||||||||||
| β1-WT | −8.29 ± 0.04 | 81.2 ± 2.1 | 24 | −9.36 ± 0.07 | 35 | −7.13 ± 0.06 | 31 | −8.69 ± 0.08 | 30 | −10.01 ± 0.06 | 33 |
| β1-A187G | −8.22 ± 0.08 | 78.9 ± 2.3 | 8 | −9.23 ± 0.08 | 14 | −7.02 ± 0.07 | 8 | −8.72 ± 0.09 | 8 | −9.99 ± 0.06 | 17 |
| β1-V189T | −8.20 ± 0.04 | 80.6 ± 1.8 | 12 | −9.26 ± 0.09 | 20 | −7.73 ± 0.07* | 15 | −8.61 ± 0.05 | 14 | −9.81 ± 0.08 | 27 |
| β1-L195Q | −8.20 ± 0.05 | 80.6 ± 2.7 | 12 | −8.97 ± 0.10 | 19 | −7.12 ± 0.07 | 15 | −8.86 ± 0.11 | 14 | −9.85 ± 0.07 | 25 |
| β1-W199Y | −8.46 ± 0.06 | 77.3 ± 2.4 | 12 | −9.66 ± 0.08 | 19 | −7.39 ± 0.07 | 16 | −9.01 ± 0.12 | 15 | −10.06 ± 0.05 | 26 |
| β1-W199A | −8.63 ± 0.07* | 85.4 ± 3.2 | 8 | −9.51 ± 0.14 | 13 | −7.26 ± 0.08 | 11 | −9.12 ± 0.11 | 13 | −10.07 ± 0.16 | 4 |
| β1-W199D | −8.91 ± 0.07* | 87.6 ± 3.5 | 8 | −9.88 ± 0.08 | 12 | −7.48 ± 0.11 | 10 | −9.17 ± 0.10 | 12 | −9.85 ± 0.12 | 8 |
| β1-W199F | −8.71 ± 0.09* | 80.0 ± 2.6 | 8 | −10.14 ± 0.10* | 14 | −7.19 ± 0.12 | 12 | −9.09 ± 0.13 | 14 | −9.95 ± 0.16 | 11 |
| β1-W199K | −7.64 ± 0.05* | 86.4 ± 3.2 | 8 | −9.46 ± 0.08 | 14 | −6.41 ± 0.06* | 9 | −8.00 ± 0.12* | 10 | a | |
| β1-W199L | −7.60 ± 0.06* | 75.2 ± 2.9 | 8 | −10.06 ± 0.11* | 12 | −6.90 ± 0.11 | 11 | −8.72 ± 0.06 | 12 | a | |
| β1-W199N | −8.88 ± 0.06* | 84.6 ± 5.4 | 8 | −9.93 ± 0.09 | 11 | −7.21 ± 0.08 | 12 | −9.22 ± 0.10 | 13 | −10.12 ± 0.12 | 12 |
| β1-V189T-L195Q | −8.22 ± 0.04 | 87.0 ± 3.4 | 8 | −9.49 ± 0.09 | 13 | −7.67 ± 0.10* | 11 | −8.81 ± 0.11 | 14 | −9.58 ± 0.09 | 12 |
| β1-V189T-W199Y | −8.62 ± 0.07* | 84.2 ± 2.1 | 8 | −9.93 ± 0.08 | 12 | −7.85 ± 0.08* | 10 | −8.80 ± 0.08 | 12 | −10.10 ± 0.15 | 11 |
| β1-L195Q-W199Y | −8.41 ± 0.06 | 79.6 ± 2.0 | 8 | −9.68 ± 0.06 | 13 | −7.24 ± 0.06 | 11 | −9.04 ± 0.11 | 12 | −9.82 ± 0.14 | 9 |
| β1-V189T-L195Q-W199Y | −8.44 ± 0.04 | 82.7 ± 2.7 | 8 | −9.85 ± 0.08 | 14 | −7.92 ± 0.06* | 11 | −8.99 ± 0.09 | 14 | −9.79 ± 0.11 | 12 |
| β1-TM4 | −8.32 ± 0.05 | 77.3 ± 1.9 | 23 | −9.43 ± 0.09 | 35 | −7.94 ± 0.06* | 31 | −9.08 ± 0.07 | 29 | −9.98 ± 0.09 | 35 |
| CGP12177 Log EC50 | % Isoprenaline | n | Log KB CGP20712A | n | Log KB ICI118551 | n | Log KB propranolol | n | |||
| CGP12177 as agonist: stable mixed populations of cells | |||||||||||
| β1-WT | −8.12 ± 0.07 | 53.3 ± 1.5 | 21 | −7.37 ± 0.08 | 37 | −5.86 ± 0.08 | 22 | −6.69 ± 0.08 | 27 | ||
| β1-A187G | −8.21 ± 0.09 | 41.5 ± 3.1 | 7 | −7.25 ± 0.11 | 12 | −5.81 ± 0.08 | 7 | −6.72 ± 0.12 | 10 | ||
| β1-V189T | −8.01 ± 0.07 | 44.1 ± 2.3 | 11 | −7.64 ± 0.08 | 21 | −6.36 ± 0.09* | 16 | −6.98 ± 0.08 | 24 | ||
| β1-L195Q | −9.00 ± 0.06* | 32.9 ± 1.4* | 11 | −8.53 ± 0.09* | 19 | −6.64 ± 0.11* | 13 | −7.74 ± 0.12* | 21 | ||
| β1-W199Y | −9.16 ± 0.05* | 40.2 ± 2.3* | 11 | −8.46 ± 0.07* | 23 | −6.69 ± 0.09* | 17 | −7.90 ± 0.09* | 29 | ||
| β1-W199A | −9.46 ± 0.11* | 68.6 ± 4.0* | 8 | −8.79 ± 0.10* | 12 | −6.88 ± 0.11* | 9 | −7.96 ± 0.15* | 9 | ||
| β1-W199D | −9.48 ± 0.08* | 45.3 ± 3.3 | 8 | −8.94 ± 0.09* | 17 | −6.98 ± 0.16* | 13 | −8.87 ± 0.12* | 20 | ||
| β1-W199F | −8.95 ± 0.13* | 58.6 ± 3.4 | 8 | −8.10 ± 0.07* | 13 | −6.53 ± 0.10* | 10 | −7.61 ± 0.17* | 12 | ||
| β1-W199K | −9.40 ± 0.05* | 85.1 ± 2.5* | 8 | −8.13 ± 0.10* | 18 | −6.34 ± 0.09* | 13 | −7.47 ± 0.08* | 20 | ||
| β1-W199L | −9.20 ± 0.06* | 82.9 ± 3.2* | 8 | −8.12 ± 0.09* | 20 | −6.45 ± 0.08* | 13 | −7.56 ± 0.11* | 20 | ||
| β1-W199N | −9.27 ± 0.12* | 47.7 ± 3.2 | 8 | −8.26 ± 0.11* | 12 | −6.67 ± 0.10* | 10 | −8.19 ± 0.09* | 17 | ||
| β1-V189T-L195Q | −9.21 ± 0.06* | 35.1 ± 2.6* | 8 | −8.64 ± 0.09* | 15 | −7.11 ± 0.08* | 12 | −8.17 ± 0.10* | 14 | ||
| β1-V189T-W199Y | −9.07 ± 0.11* | 43.2 ± 2.9 | 8 | −8.70 ± 0.08* | 18 | −7.42 ± 0.14* | 13 | −7.82 ± 0.10* | 16 | ||
| β1-L195Q-W199Y | −9.51 ± 0.06* | 41.3 ± 2.3 | 8 | −9.17 ± 0.12* | 15 | −6.94 ± 0.09* | 12 | −8.15 ± 0.11* | 15 | ||
| β1-V189T-L195Q-W199Y | −9.40 ± 0.07* | 39.2 ± 2.4* | 8 | −9.21 ± 0.12* | 14 | −7.49 ± 0.07* | 12 | −8.66 ± 0.11* | 16 | ||
| β1-TM4 | −9.43 ± 0.06* | 33.9 ± 1.5* | 19 | −8.98 ± 0.07* | 39 | −7.64 ± 0.08* | 32 | −8.39 ± 0.06* | 40 | ||
CGP12177 is very efficacious in these mutants, similar to cimaterol, and it is therefore not possible to measure a log KB value for CGP12177 by a rightward shift of the cimaterol concentration response.
P < 0.001 One-way ANOVA with post hoc Newman–Keuls comparing values from the mutant receptors with those obtained from the β1-WT, thus the log KB for ICI118551 at β1-V189T is different from that obtained from the β1-WT with P < 0.001
TABLE 9.
The ratio of the log KB value for CGP12177 (as an antagonist of cimaterol; thus, at the high-affinity conformation) and log EC50 value for CGP12177 (as an agonist of the secondary low-affinity conformation) for responses seen at β1-WT, β1-TM4, and β1-receptors with point mutations in TM4 (data taken from Table 8)
Ratio of the affinity for antagonists at the two conformations is also given (i.e., log KB for antagonism of cimaterol at high affinity site/log KB for antagonism of CGP12177 at low affinity site). This table examines the ratios of the antagonist affinities and shows that the difference in affinity between the antagonists at the catecholamine conformation (in the presence of cimaterol) and secondary conformation (presence of CGP12177) is substantially less with the L195 and W199 mutations, suggesting that these are important for the secondary conformation. Although V189T alters the affinity of ICI118551, the ratio of affinity does not change, suggesting that V189T does not have a major role in the secondary conformation.
| Ratio of Log KB CGP12177/ Log EC50 CGP12177 | Ratio of Log KB (Cimaterol as Agonist)/Log KB (CGP12177 as Agonist) |
|||
|---|---|---|---|---|
| CGP20712A | ICI118551 | Propranolol | ||
| β1-WT | 1.89 | 1.99 | 1.27 | 2.00 |
| β1-A187G | 1.78 | 1.98 | 1.21 | 2.00 |
| β1-V189T | 1.80 | 1.62 | 1.37 | 1.63 |
| β1-L195Q | 0.85 | 0.44 | 0.48 | 1.12 |
| β1-W199Y | 0.90 | 1.20 | 0.70 | 1.11 |
| β1-W199A | 0.61 | 0.72 | 0.38 | 1.16 |
| β1-W199D | 0.37 | 0.94 | 0.50 | 0.30 |
| β1-W199F | 1.00 | 2.04 | 0.66 | 1.48 |
| β1-W199K | a | 1.33 | 0.07 | 0.53 |
| β1-W199L | a | 1.94 | 0.45 | 1.16 |
| β1-W199N | 0.85 | 1.67 | 0.54 | 1.03 |
| β1-V189T-L195Q | 0.64 | 0.85 | 0.56 | 0.64 |
| β1-V189T-W199Y | 1.03 | 1.23 | 0.43 | 0.98 |
| β1-L195Q-W199Y | 0.31 | 0.51 | 0.30 | 0.89 |
| β1-V189T-L195Q-W199Y | 0.39 | 0.64 | 0.43 | 0.33 |
| β1-TM4 | 0.55 | 0.45 | 0.30 | 0.69 |
Fig. 3.
CRE-SPAP production in response to cimaterol in the absence and presence of 10 nM and 100 nM CGP20712A and 1µM ICI118551 in stable mixed populations of cell lines expressing (A) β1-WT, (B) β1-V189T, (C) β1-L195Q, (D) β1-W199Y, and (E) β1-TM4. Bars represent basal CRE-SPAP production, that in response to 10 µM isoprenaline or that in response to 10 nM or 100 nM CGP20712A or 1 µM ICI118551 alone. Data points are mean ± S.E.M. of triplicate determinations. These single experiments are representative of (A) 24, (B) 12, (C) 12, (D) 12, and (E) 23 separate experiments. This figure shows that CGP20712A inhibits cimaterol responses in all of these receptors with similar ability; however, ICI118551 is able to inhibit β1-V189T and β1-TM4 to a greater extent than the others.
Fig. 4.
CRE-SPAP production in response to CGP12177 in the absence and presence of 100 nM and 1 µM CGP20712A and 10 µM ICI118551 in stable mixed populations of cell lines expressing (A) β1-WT, (B) β1-V189T, (C) β1-L195Q, (D) β1-W199Y, and (E) β1-TM4. Bars represent basal CRE-SPAP production, that in response to 10 µM isoprenaline or that in response to 100 nM or 1 µM CGP20712A or 10 µM ICI118551 alone. Data points are mean ± S.E.M. of triplicate determinations. These single experiments are representative of (A) 21, (B) 11, (C) 11, (D) 11, and (E) 20 separate experiments. This figure shows that secondary conformation CGP12177 responses are more readily inhibited by CGP20712A and ICI118551 at β1-L195Q, β1-W199Y, and β1-TM4 than at β1-WT, suggesting that the secondary conformation is compromised in the β1-L195Q, β1-W199Y, and β1-TM4 mutants. β1-V189T, while remaining more resistant to CGP20712A inhibition (i.e., demonstrating preservation of the secondary conformation), is once again more sensitive to ICI118551 inhibition.
We also examined the impact of these specific mutations in combination with each other to determine whether the effects of each change were additive or whether one single change was sufficient to account for the secondary site. A combination of L195Q and W199Y largely achieved the same effect as the whole TM4 swap, with the exception of the affinity of ICI118551 (Table 8). However, addition of the V189T mutation (thus making a triple β1-V189T-L195Q-W199Y) effectively normalized the affinity of ICI118551 to that observed with the full TM4 swap (Fig. 5, A and B). This suggests that β1-V189T-L195Q-W199Y has also lost the secondary conformation.
Fig. 5.
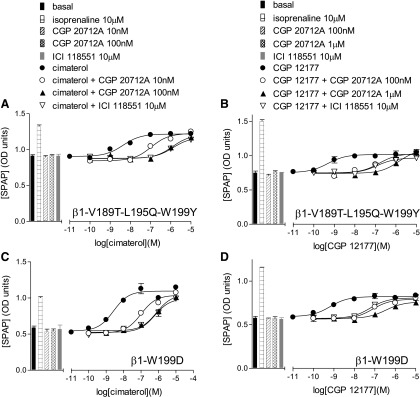
CRE-SPAP production in response to cimaterol (A and C) and CGP12177 (B and D) in the absence and presence of 10 nM, 100 nM, or 1 µM CGP20712A and 10 µM ICI118551 in stable mixed populations of cell lines expressing β1-V189T-L195Q-W199Y (A and B) or β1-W199D (C and D). Bars represent basal CRE-SPAP production, that in response to 10 µM isoprenaline or that in response to 10 nM, 100 nM, or 1 µM CGP20712A or 10 µM ICI118551 alone. Data points are mean ± S.E.M. of triplicate determinations. These single experiments are representative of eight separate experiments in each case. This figure shows that CGP20712A and ICI118551 are able to inhibit cimaterol and CGP12177 responses to a similar degree at both β1-V189T-L195Q-W199Y and β1-W199D, unlike that seen at β1-WT (Figs. 3A and 4A). This suggests that while both are primarily β1-adrenoceptors (given that both are more readily inhibited by CGP20712A than ICI118551) the secondary conformation is compromised in both of these mutant receptors.
In addition to the simple substitution of β2-adrenoceptor residues for their equivalents in the β1-adrenoceptor, we also examined the effects of different substitutions at position 199. Thus at position 199, as well as mutating the tryptophan (W) to tyrosine (Y, as in the human β2-adrenoceptor) it was also mutated to another aromatic residue (phenylalanine, F), a hydrophobic residue (leucine, L), a basic residue (lysine, K), an amide (asparagine, N), an acid (aspartic acid, D) and the small amino acid alanine (A). Substitution of W for other residues had little effect on the nM EC50 for CGP12177 observed with the tyrosine (Y) residue from the β2-adrenoceptor, and most appeared to have lost, or at least compromised, the secondary conformation (Fig. 5, C and D; Table 8). Interestingly, the W199K and W199L mutations decreased the EC50 of cimaterol (top half of Table 8) while increasing the efficacy of CGP12177 which then appeared as a virtual full agonist in these two mutations (bottom half of Table 8).
Confirmation of Loss of the Secondary Site with Mutations V189T, L195Q, and W199Y: 3H-cAMP Accumulation in Transiently Transfected Cells.
So far, identification of the role of amino acids at positions 195, 199, and possibly 189 in the secondary conformation pharmacology of the β1-adrenoceptor has relied on observation of 1) different affinity values for antagonists in the presence of cimaterol and CGP12177, and 2) the comparison between the partial agonist log EC50 value and log KB value for CGP12177. To confirm that these amino acids were indeed important, evidence was sought using a different assay to explore two further strands of evidence: the stimulation resulting from CGP12177 in the presence of a fixed concentration of cimaterol and the effect on the response to pindolol. To examine whether cimaterol and CGP12177 were competing at the same site of the receptor, CGP12177 concentration responses were examined in the presence of different fixed concentrations of cimaterol. Low concentrations of CGP12177 competed with cimaterol causing a reduction in the stimulatory response. However, higher CGP12177 concentrations stimulated responses, creating a “dip” in the curve, for the two conformation β1-WT adrenoceptor (Pak and Fishman, 1996; Fig. 6A). This “dip” was absent in the single conformation β2-WT adrenoceptor and at β1-TM4 (Fig. 6, C and E; Table 10). The β1-V189T mutation alone had little effect on the two conformations (Fig. 7A; Table 10), although the “dip” appeared reduced in both L195Q and W199Y mutations alone. However, the “dip” was absent from β1-V189T-L195Q-W199Y and β1-W199D (Fig. 8, A and C; Table 10) in keeping with a single conformation receptor. Interestingly, in the transiently transfected cells, the β1-WT 3H-cAMP accumulation response to CGP12177 was best described by a two-component response (Fig. 6A; Table 10). Hints that the CGP12177 response may, at times, be biphasic have been seen before (e.g., Fig. 4 of Baker et al., 2013) and are also potentially present in the stable β1-WT cell lines and stable mixed β1-WT population data (Figs. 1B and 4A, respectively). Interestingly, mutations L195Q and W199Y (or W199D) removed the biphasic response of CGP12177 (Figs. 7, C and E, and 8C; Table 10). Finally, concentration responses to the biphasic ligand pindolol were examined (Figs. 6, B, D and F, 7, B, D, and F, and 8, B and D; Table 10). Both the racemic and S-isomer of this ligand have previously been shown to stimulate agonist responses via both conformations of the β1-adrenoceptor (Walter et al., 1984; Baker, 2010a). The data obtained with this agonist were consistent with the observations made with CGP12177 (apart from W199Y, which continued to exhibit two-site pharmacology with pindolol; Table 10). However, this was not the case with W199D or combinations of W199Y with L195Q and/or V189T (Table 10).
TABLE 10.
Log EC50 values and % maximum response to isoprenaline for 3H-cAMP accumulation in response to CGP12177 and pindolol at β1-WT, β2-WT, and β1-adrenoceptors with single point mutations or combinations of these mutations
These data were obtained from transiently transfected cells, and n refers to the number of separate experiments, each conducted in a separate transiently transfected population of cells. This table shows that the biphasic responses to CGP12177 and pindolol, and the “dip” in the curve seen when CGP12177 competed with a fixed concentration of cimaterol (see Figs. 6–8) observed in the β1-WT, are lost with several β1-TM4 mutant receptors, including the β1-L195Q-W199Y, β1-V189T-L195Q-W199Y, and β1-W199D receptors.
| CGP12177 as Agonist: Transiently Transfected Cells | Pindolol as Agonist: Transiently Transfected Cells | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Log EC150 |
Log EC250 |
% Site 1 |
% Max Isoprenaline |
Competition with Cimaterol (Dip) |
n |
Log EC150 |
Log EC250 |
% Site 1 |
% Max Isoprenaline |
n |
|
| β1-WT | −9.28 ± 0.17 | −7.36 ± 0.21 | 42.3 ± 7.2 | 53.0 ± 2.9 | Yes | 6 | −9.10 ± 0.04 | −5.93 ± 0.18 | 57.1 ± 3.3 | 30.0 ± 2.0 | 8 |
| β1-V189T | −9.37 ± 0.12 | −7.16 ± 0.06 | 45.1 ± 2.7 | 48.4 ± 4.6 | Yes | 6 | −8.87 ± 0.05 | −5.78 ± 0.14 | 67.1 ± 3.3 | 22.3 ± 2.9 | 5 |
| β1-L195Q | −9.02 ± 0.09 | 37.4 ± 1.5 | Slight | 3 | −9.14 ± 0.08 | 27.9 ± 1.6 | 3 | ||||
| β1-W199Y | −9.28 ± 0.11 | 41.0 ± 3.2 | Slight | 4 | −9.02 ± 0.21 | −6.49 ± 0.18 | 71.7 ± 1.4 | 32.4 ± 3.0 | 4 | ||
| β1-W199D | −9.80 ± 0.06 | 49.9 ± 1.0 | No | 6 | −8.96 ± 0.23 | 28.9 ± 1.8 | 4 | ||||
| β1-L195Q-W199Y | −9.62 ± 0.05 | 39.9 ± 3.7 | No | 4 | −9.33 ± 0.08 | 26.2 ± 3.0 | 4 | ||||
| β1-V189T-L195Q-W199Y | −9.52 ± 0.05 | 35.3 ± 6.3 | No | 3 | −9.11 ± 0.03 | 21.8 ± 1.4 | 4 | ||||
| β1-TM4 | −9.54 ± 0.06 | 30.9 ± 1.8 | No | 4 | −9.25 ± 0.08 | 23.0 ± 3.1 | 3 | ||||
| β2-WT | −9.81 ± 0.15 | 11.2 ± 1.6 | No | 4 | −9.58 ± 0.06 | 11.6 ± 0.4 | 4 | ||||
Fig. 7.
3H-cAMP accumulation in response to CGP12177 (A, C, and E), and pindolol (B, D, and F) in transiently transfected cells expressing β1-V189T (A and B), β1-L195Q (C and D), and β1-W199Y (E and F). The CGP12177 responses were examined in the absence and presence of 3, 10, and 30 nM cimaterol. Bars represent basal 3H-cAMP accumulation, that in response to 10 µM isoprenaline or that in response to 3, 10, and 30 nM cimaterol alone. Data points are mean ± S.E.M. of triplicate determinations. These single experiments are representative of (A) six, (B) five, (C) three, (D) three, (E) four, and (F) four separate experiments. This figure shows that both the “dip” in the CGP12177 + cimaterol curve and biphasic pindolol response are still present at the β1-V189T, suggesting conservation of the secondary conformation. However, both are reduced at β1-L195Q and β1-W199Y, suggesting some loss of the secondary conformation at β1-L195Q and β1-W199Y (see Fig. 6A for β1-WT comparison).
Fig. 8.
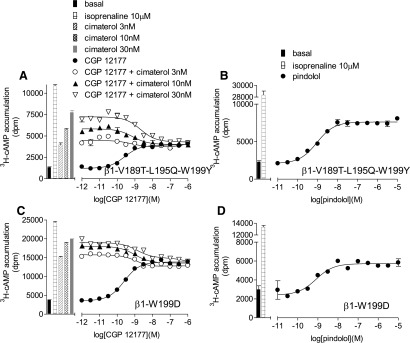
3H-cAMP accumulation in response to CGP12177 (A and C) and pindolol (B and D) in transiently transfected cells expressing β1-V189T-L195Q-W199Y (A and B) and β1-W199D (C and D). The CGP12177 responses were examined in the absence and presence of 3, 10, and 30 nM cimaterol. Bars represent basal 3H-cAMP accumulation, that in response to 10 µM isoprenaline or that in response to 3, 10, and 30 nM cimaterol alone. Data points are mean ± S.E.M. of triplicate determinations. These single experiments are representative of (A) three, (B) four, (C) six, and (D) four separate experiments. This figure shows that the “dip” in the CGP12177 + cimaterol curve and biphasic pindolol response, both present at β1-WT with two conformations (see Fig. 6A), are absent in β1-V189T-L195Q-W199Y and β1-W199D, suggesting that neither of these receptors has the secondary conformation.
Discussion
The human β1-WT exists in at least two agonist conformations: a high-affinity catecholamine conformation and a low-affinity secondary (CGP12177) conformation (Pak and Fishman, 1996; Kaumann and Molenaar, 2008). This secondary conformation does not occur in the closely related human β2-WT (Pak and Fishman, 1996; Baker et al., 2002). This study attempted to identify the key area responsible for this secondary conformation using a β1/β2-chimeric receptor approach (Isogaya et al., 1999; Kaumann and Molenaar 2008).
At the β1-WT, the secondary conformation was clearly delineated from the primary catecholamine conformation by two pieces of evidence. Firstly, the cimaterol responses (catecholamine conformation agonist) were readily inhibited by antagonists, whereas CGP12177 responses required much greater antagonist concentrations, yielding lower log KB values (Table 2). Secondly, CGP12177 readily inhibited the cimaterol responses at low concentrations (log KB −9.55), but required high concentrations to produce its agonist response (log EC50 −8.18), in keeping with previous studies (e.g., Pak and Fishman, 1996; Lowe et al., 2002; Baker et al., 2003; Joseph et al., 2004a; Baker, 2005a). At the β2-WT, cimaterol and CGP12177 responses were inhibited by similar concentrations of antagonist and the EC50/KB discrepancy for CGP12177 was less marked, suggesting competition of the two ligands at the same conformation.
When the TM-chimeras were examined, antagonism of cimaterol responses suggested that all receptors (β1-TM1 to β1-TM7 and β2-TM1 to β2-TM7; Tables 2–5) retained their respective pharmacological parent β1 or β2-subtype, even when one entire transmembrane region was altered (i.e., high affinity for CGP20712A at β1-adrenoceptors, high affinity for ICI118551 at β2-adrenoceptors). Significant changes, however, were seen in the secondary conformation in β1-TM4. Here, antagonists inhibited the cimaterol and CGP12177 responses to give similar log KB values (Tables 4 and 5), and the concentration of CGP12177 required to inhibit the cimaterol response (−9.63) approached that required to stimulate the agonist response (−9.27). Taken together this strongly suggests that the secondary conformation is absent in β1-TM4. Although direct comparison of partial agonist efficacy across cell lines is difficult, the log EC50 for CGP12177 (−9.27) is substantially more potent (left-shifted) at β1-TM4 than any other β1-TM chimeric receptor (−7.83 to −8.27; Tables 2 and 3) even though the response is less efficacious (% isoprenaline maximum response achieved) and the receptor expression level is the lowest of the β1 cell lines. If CGP12177 were simply more efficacious at β1-TM4, the left-shifted EC50 would be accompanied by an increase in the maximum response obtained. The increase in potency, but decrease in efficacy, suggests that something different is happening at β1-TM4, and indeed these values are similar to those obtained for CGP12177 at the β2-WT and β2-chimeric receptors (Tables 2–5). This differs from the comment in Kaumann and Molenaar (2008) regarding TM5.
Thus, β1-TM4 appears to be a β1-adrenoceptor without the secondary conformation. When the β2-chimeras were examined (Tables 4 and 5), the antagonist affinities (whether cimaterol or CGP12177 was the agonist), log EC50, and log KB values for CGP12177 were similar for all β2-chimeric receptors to those of β2-WT and β1-TM4. Thus, although the secondary site was lost in β1-WT, it was not recreated in β2-WT by the TM4 swap, and, therefore, other areas of the receptor must contribute to this secondary conformation.
To locate the specific amino acids involved in the secondary conformation, intermediate stages in the generation of β1-TM4 were examined. The log EC50 for CGP12177 was more potent and the antagonist affinities were higher for β1-TM4 stage 5 and the full β1-TM4 chimeric receptor (Tables 1, 6, and 7). ICI118551 had a slightly different pattern with increased affinity at both conformations from β1-TM4 stage 4 onwards. This suggests that amino acids L195, W199, and maybe V189 are important for the secondary conformation. These mutations were therefore examined alone. For β1-L195Q and β1-W199Y, the antagonist affinities (log KB) in the presence of CGP12177 were higher, and the log EC50 for CGP12177 more potent, than at β1-WT, suggesting that both of these residues are important in the secondary conformation. ICI118551 had higher affinity for β1-V189T in the presence of both cimaterol and CGP12177, suggesting all-around (not conformation specific) higher affinity. This was seen in all receptors containing a V189T mutation (i.e., β1-TM4, β1-V189T, β1-V189T-L195Q, β1-V189T-W199Y, and β1-V189T-L195Q-W199Y). ICI118551 also showed lower affinity at β2-TM4 (containing the reverse T164V mutation). V189T is therefore specifically involved in the affinity of ICI118551. CGP20712A and propranolol both had slightly higher affinity in the presence of CGP12177 at β1-V189T than β1-WT, giving rise to lower ratios (Table 9) and suggesting a potential minor role for V189T in the secondary conformation.
However, neither L195Q nor W199Y alone gave KB or EC50 values that matched those of the β1-TM4 (Tables 8 and 9). Double and triple mutations were therefore made. At β1-V189TL-195Q-W199Y, antagonist affinities and log EC50 values for CGP12177 were the same as for the β1-TM4, suggesting complete abolishment of the secondary conformation. β1-L195Q-W199Y also achieved this (with the exception of ICI118551 affinity because this lacks the V189T mutation). β1-V189T-L195Q and β1-V189T-W199Y both had the higher ICI118551 affinity, but the secondary conformation affinity for CGP20712A and propranolol did not reach that of β1-TM4. This suggests that while L195Q and W199Y are each important, their effects are additive and that both are needed to completely abolish the secondary conformation. The effect of V189T, while important for ICI118551 affinity, was minor for the secondary conformation.
The effect of charge at the W199 site was then assessed. The addition of a negatively charged group (W199D) greatly increased CGP20712A and propranolol affinity in the presence of CGP12177 to that of β1-TM4, β1-L195Q-W199Y, and β1-V189T-L195Q-W199Y, suggesting that this single change could remove the secondary conformation (although ICI118551 affinity does not reach that of β1-TM4 or β1-V189T-L195Q-W199Y because V189T is missing). Thus, the single amino acid change of W199D appeared to create a β1-adrenoceptor with β1 pharmacological characteristics (i.e., 100-fold higher affinity for CGP20712A than ICI118551) but that is completely devoid of the secondary conformation.
Finally, to confirm L195Q, W199Y, (and maybe V189T) involvement in the secondary conformation, 3H-cAMP accumulation was investigated (Figs. 6–8). At low concentrations, CGP12177 inhibited the stimulation of the catecholamine conformation by cimaterol, while at higher concentrations CGP12177 stimulated the secondary conformation, resulting in a “dip” in the response to cimaterol in the presence of CGP12177 (Fig. 6A) (Pak and Fishman, 1996; Konkar et al., 2000; Baker et al., 2003; Baker 2005a). This “dip” was present for β1-V189T (Fig. 7A) but reduced at β1-L195Q and β1-W199Y (Fig. 7, C and E). However, there was no “dip” in β2-WT, β1-TM4, β1-L195Q-W199Y, β1-V189T-L195Q-W199Y, or β1-W199D (Figs. 6, C and E, and 8, A and C). At all these receptors, CGP12177 competed with cimaterol to inhibit the cimaterol response at the same as (or just to the right of) that of the EC50 value, strongly suggesting competition at the same conformation.
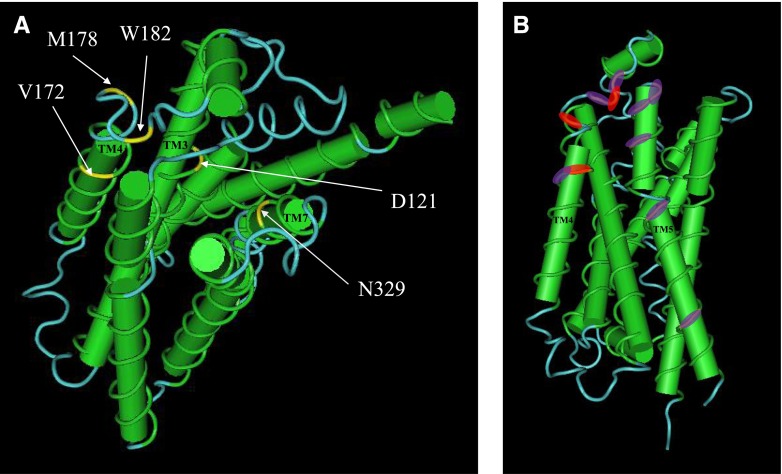
Pindolol has a biphasic concentration response curve at the β1-adrenoceptor. The first (higher affinity) component is thought to occur via the catecholamine conformation, while the second component via the secondary conformation (Walter et al., 1984; Baker et al., 2003). Therefore, the pindolol response should become a single-component response at β1-TM4 and the other receptors lacking the secondary conformation. Indeed, pindolol was found to have a two-component concentration response curve at β1-WT and β1-V189T, but a single-component response curve at β2-WT, β1-TM4, β1-L195Q-W199Y, β1-T189V-L195Q-W199Y, and β1-W199D, confirming the crucial roles of L195 and W199 in the secondary conformation. Interestingly, for all β-adrenoceptors with a known sequence and two pharmacologically confirmed conformations, V, L, and W, or similar, are found at equivalent positions for 189, 195, and 199, respectively, while those of one-conformation β-adrenoceptors have T, Q, and Y (Table 11).
TABLE 11.
Amino acids occurring at positions 189, 195, and 199 or equivalent positions in other β-adrenoceptors from several species
| UniProtKB/TrEMBL Accession Number |
Position 189 |
Position 195 |
Position 199 |
|
|---|---|---|---|---|
| Two-conformation receptors | ||||
| Human β1 | P08588 | V 189 | L 195 | W 199 |
| Rat β1 | P18090 | V 189 | L 195 | W 199 |
| Mouse β1 | P34971 | V 189 | L 195 | W 199 |
| Guinea pig β1 | B0FL73 | V 189 | L 195 | W 199 |
| Cat β1 | Q9TST6 | V 189 | L 195 | W 199 |
| Turkey β1 | P07700 | V 172 | M 178 | W 182 |
| Turkey β4C | P43141 | I 154 | M 160 | W 164 |
| Human β3 | P13945 | V 168 | M 174 | W 178 |
| One-conformation receptors | ||||
| Human β2 | P07550 | T 164 | Q 170 | Y 174 |
| Turkey β3C | a | T 161 | Q 167 | Y 171 |
Sequence from Fig. 1 in Baker, 2010b.
A recent turkey β1-adrenoceptor crystal structure suggests that TM4 is involved in a β1-dimer interface with TM5 (Huang et al., 2013). Figure 9A shows that the equivalent key turkey TM4 residues (V172, M178, and W182; Table 11) are largely outward facing, away from the binding pocket, but lie within the TM4/TM5 dimer interface (Fig. 9B). However, the key binding residues in TM3 (D138) and TM7 (N363), that are essential for both the catecholamine and secondary conformation responses (Baker et al., 2008), are both inward-facing residues within the ligand binding pocket (Fig. 9A). It is therefore interesting to speculate that both binding site activation and receptor dimerization are involved in the secondary conformation. However, more work is required to understand how and where molecules bind to create the distinct pharmacological patterns observed.
Fig. 9.
Location of equivalent key residues (V172, M178, W182) in the crystal structure of the turkey β1-adrenoceptor described by Huang et al. (2013). This structure was obtained in the ligand-free state and in a lipid membrane–like environment and suggested that TM4 was involved in a dimer interface with TM5. Views of one protomer of the oligomeric structure (PDB ID 4GPO) reported by Huang et al. (2013) were generated with Cn3D (National Center for Biotechnology Information). (A) Residues V172, M178, and W182 (equivalent to the human V189, L195, and W199) and D121 and N329 (equivalent to D138 and N363, identified in a previous study as essential for both catecholamine and secondary conformation functional responses) (Baker et al., 2008) are highlighted in yellow. (B) View of V172, M178, and W182 (highlighted in red) with the key residues for dimerization in TM5 (R205, A206, A210, I218, R229), TM4 (L171), and the proximal region of extracellular loop 2 (W181, R183) identified by Huang et al. (2013) highlighted in purple. Interestingly, V172 and W182 are on the same outward facing surface as the key dimerization residues, with W182 sandwiched between the two residues (W181, R183), and V172 is next to another (L171) identified by Huang et al. (2013).
In conclusion, the β1-adrenoceptor exists in two agonist conformations, and the key residues responsible for the secondary low-affinity conformation are in TM4. The mutation β1-V189T has an effect on the affinity of ICI118551 and may have a minor role in secondary conformation pharmacology. However, the amino acids at positions 195 and 199 in TM4 both have a major role in the secondary conformation, and together L195Q and W199Y (or indeed W199D alone), abolish this secondary conformation and create a β1-adrenoceptor with only one high-affinity agonist conformation. From Huang et al. (2013) data, it is tempting to speculate that oligomerization involving TM4 may underlie the secondary β1-adrenoceptor conformation.
Acknowledgments
The authors thank Nicola Hawley and Anne Webber for technical assistance with molecular biology, and June McCulloch for assistance with the 3H-cAMP chromatography columns.
Abbreviations
- CHO
Chinese hamster ovary
- CGP12177
(−)-4-(3-tert-butylamino-2-hydroxypropoxy)-benzimidazol-2-one
- CGP20712A
2-hydroxy-5-(2-[{hydroxy-3-(4-[1-methyl-4-trifluoromethyl-2-imidazolyl]phenoxy)propyl}amino]ethoxy)benzamide
- CRE-SPAP
cAMP response element upstream of a secreted placental alkaline phosphatase reporter gene
- ICI118551
(−)-1-(2,3-[dihydro-7-methyl-1H-inden-4-yl]oxy)-3-([1-methylethyl]-amino)-2-butanol
- KB
half receptor occupancy
- sfm
serum free media
- TM
transmembrane
- WT
wild-type
Authorship Contributions
Participated in research design: Baker.
Conducted experiments: Baker, Proudman.
Contributed new reagents or analytic tools: Baker, Proudman, Hill.
Performed data analysis: Baker.
Wrote or contributed to the writing of the manuscript: Baker, Hill.
Footnotes
This work was supported by a Wellcome Trust Clinician Scientist Fellowship awarded to J.G.B. [Grant 073377/Z/03/Z].
References
- Arch JR. (2004) Do low-affinity states of beta-adrenoceptors have roles in physiology and medicine? Br J Pharmacol 143:517–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Hall IP, Hill SJ. (2002) Pharmacological characterization of CGP 12177 at the human β(2)-adrenoceptor. Br J Pharmacol 137:400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG. (2005a) Site of action of β-ligands at the human β1-adrenoceptor. J Pharmacol Exp Ther 313:1163–1171 [DOI] [PubMed] [Google Scholar]
- Baker JG. (2005b) Evidence for a secondary state of the human β3-adrenoceptor. Mol Pharmacol 68:1645–1655 [DOI] [PubMed] [Google Scholar]
- Baker JG. (2010a) The selectivity of β-adrenoceptor agonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol 160:148–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG. (2010b) A full pharmacological analysis of the three turkey β-adrenoceptors and comparison with the human β-adrenoceptors. PLoS ONE 5:e15487 10.1371/Journal.pone.0015487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Hall IP, Hill SJ. (2003) Agonist actions of “β-blockers” provide evidence for two agonist activation sites or conformations of the human β1-adrenoceptor. Mol Pharmacol 63:1312–1321 [DOI] [PubMed] [Google Scholar]
- Baker JG, Proudman RGW, Hawley NC, Fischer PM, Hill SJ. (2008) Role of key transmembrane residues in agonist and antagonist actions at the two conformations of the human β1-adrenoceptor. Mol Pharmacol 74:1246–1260 [DOI] [PubMed] [Google Scholar]
- Baker JG, Proudman RGW, Hill SJ. (2013) Impact of polymorphic variants on the molecular pharmacology of the two-agonist conformations of the human β1-adrenoceptor. PLoS ONE 8:e77582 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman JG. (2001) The putative beta4-adrenergic receptor is a novel state of the beta1-adrenergic receptor. Am J Physiol Endocrinol Metab 280:E199–E202 [DOI] [PubMed] [Google Scholar]
- Huang J, Chen S, Zhang JJ, Huang XY. (2013) Crystal structure of oligomeric β1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat Struct Mol Biol 20:419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogaya M, Sugimoto Y, Tanimura R, Tanaka R, Kikkawa H, Nagao T, Kurose H. (1999) Binding pockets of the beta(1)- and beta(2)-adrenergic receptors for subtype-selective agonists. Mol Pharmacol 56:875–885 [DOI] [PubMed] [Google Scholar]
- Joseph SS, Lynham JA, Molenaar P, Grace AA, Colledge WH, Kaumann AJ. (2003) Intrinsic sympathomimetic activity of (-)-pindolol mediated through a (-)-propranolol-resistant site of the beta1-adrenoceptor in human atrium and recombinant receptors. Naunyn Schmiedebergs Arch Pharmacol 368:496–503 [DOI] [PubMed] [Google Scholar]
- Joseph SS, Lynham JA, Colledge WH, Kaumann AJ. (2004a) Binding of (-)-[3H]-CGP12177 at two sites in recombinant human beta 1-adrenoceptors and interaction with beta-blockers. Naunyn Schmiedebergs Arch Pharmacol 369:525–532 [DOI] [PubMed] [Google Scholar]
- Joseph SS, Colledge WH, Kaumann AJ. (2004b) Aspartate138 is required for the high-affinity ligand binding site but not for the low-affinity binding site of the beta1-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol 370:223–226 [DOI] [PubMed] [Google Scholar]
- Kaumann AJ. (1973) Adrenergic receptors of cardiac muscle. Two different mechanisms of β-blockers as partial agonists. Acta Physiol Lat Am 23:619–620 [PubMed] [Google Scholar]
- Kaumann AJ. (1983) Cardiac β receptors—experimental aspects [in German]. Z Kardiol 72:63–82 [PubMed] [Google Scholar]
- Kaumann AJ, Blinks JR. (1980) beta-Adrenoceptor blocking agents as partial agonists in isolated heart muscle: dissociation of stimulation and blockade. Naunyn Schmiedebergs Arch Pharmacol 311:237–248 [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Engelhardt S, Hein L, Molenaar P, Lohse M. (2001) Abolition of (-)-CGP 12177-evoked cardiostimulation in double beta1/beta2-adrenoceptor knockout mice. Obligatory role of beta1-adrenoceptors for putative beta4-adrenoceptor pharmacology. Naunyn Schmiedebergs Arch Pharmacol 363:87–93 [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Molenaar P. (2008) The low-affinity site of the beta1-adrenoceptor and its relevance to cardiovascular pharmacology. Pharmacol Ther 118:303–336 [DOI] [PubMed] [Google Scholar]
- Kompa AR, Summers RJ. (1999) Desensitization and resensitization of beta 1- and putative beta 4-adrenoceptor mediated responses occur in parallel in a rat model of cardiac failure. Br J Pharmacol 128:1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkar AA, Zhu Z, Granneman JG. (2000) Aryloxypropanolamine and catecholamine ligand interactions with the β(1)-adrenergic receptor: evidence for interaction with distinct conformations of β(1)-adrenergic receptors. J Pharmacol Exp Ther 294:923–932 [PubMed] [Google Scholar]
- Kozłowska H, Schlicker E, Kozłowski M, Baranowska M, Malinowska B. (2006) Potential involvement of a propranolol-insensitive atypical beta-adrenoceptor the vasodilator effect of cyanopindolol in the human pulmonary artery. J Physiol Pharmacol 57:317–328 [PubMed] [Google Scholar]
- Kozłowska H, Szymska U, Schlicker E, Malinowska B. (2003) Atypical beta-adrenoceptors, different from beta 3-adrenoceptors and probably from the low-affinity state of beta 1-adrenoceptors, relax the rat isolated mesenteric artery. Br J Pharmacol 140:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MD, Lynham JA, Grace AA, Kaumann AJ. (2002) Comparison of the affinity of β-blockers for two states of the β 1-adrenoceptor in ferret ventricular myocardium. Br J Pharmacol 135:451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska B, Schlicker E. (1996) Mediation of the positive chronotropic effect of CGP 12177 and cyanopindolol in the pithed rat by atypical beta-adrenoceptors, different from beta 3-adrenoceptors. Br J Pharmacol 117:943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallem MY, Toumaniantz G, Serpillon S, Gautier F, Gogny M, Desfontis JC, Gauthier C. (2004) Impairment of the low-affinity state beta1-adrenoceptor-induced relaxation in spontaneously hypertensive rats. Br J Pharmacol 143:599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P. (2003) The ‘state’ of beta-adrenoceptors. Br J Pharmacol 140:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P, Chen L, Semmler AB, Parsonage WA, Kaumann AJ. (2007) Human heart beta-adrenoceptors: beta1-adrenoceptor diversification through ‘affinity states’ and polymorphism. Clin Exp Pharmacol Physiol 34:1020–1028 [DOI] [PubMed] [Google Scholar]
- Pak MD, Fishman PH. (1996) Anomalous behavior of CGP 12177A on beta 1-adrenergic receptors. J Recept Signal Transduct Res 16:1–23 [DOI] [PubMed] [Google Scholar]
- Sarsero D, Russell FD, Lynham JA, Rabnott G, Yang I, Fong KM, Li L, Kaumann AJ, Molenaar P. (2003) (-)-CGP 12177 increases contractile force and hastens relaxation of human myocardial preparations through a propranolol-resistant state of the beta 1-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol 367:10–21 [DOI] [PubMed] [Google Scholar]
- Stephenson RP. (1956) A modification of receptor theory. Br Pharmacol Chemother 11:379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin M, Simons P, Jaeggi K, Wigger N. (1983) CGP-12177. A hydrophilic beta-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem 258:3496–3502 [PubMed] [Google Scholar]
- Sawangkoon S, Miyamoto M, Nakayama T, Hamlin RL. (2000) Acute cardiovascular effects and pharmacokinetics of carvedilol in healthy dogs. Am J Vet Res 61:57–60 [DOI] [PubMed] [Google Scholar]
- Walter M, Lemoine H, Kaumann AJ. (1984) Stimulant and blocking effects of optical isomers of pindolol on the sinoatrial node and trachea of guinea pig. Role of beta-adrenoceptor subtypes in the dissociation between blockade and stimulation. Naunyn Schmiedebergs Arch Pharmacol 327:159–175 [DOI] [PubMed] [Google Scholar]
- Zakrzeska A, Schlicker E, Kwolek G, Kozłowska H, Malinowska B. (2005) Positive inotropic and lusitropic effects mediated via the low-affinity state of beta1-adrenoceptors in pithed rats. Br J Pharmacol 146:760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]