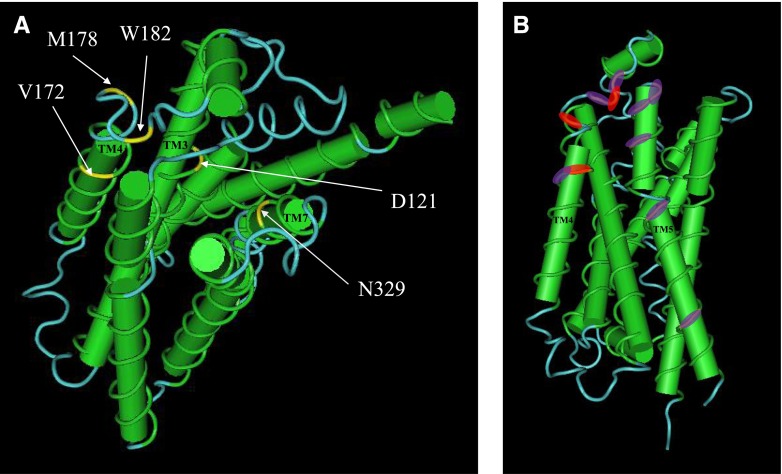
Fig. 9.
Location of equivalent key residues (V172, M178, W182) in the crystal structure of the turkey β1-adrenoceptor described by Huang et al. (2013). This structure was obtained in the ligand-free state and in a lipid membrane–like environment and suggested that TM4 was involved in a dimer interface with TM5. Views of one protomer of the oligomeric structure (PDB ID 4GPO) reported by Huang et al. (2013) were generated with Cn3D (National Center for Biotechnology Information). (A) Residues V172, M178, and W182 (equivalent to the human V189, L195, and W199) and D121 and N329 (equivalent to D138 and N363, identified in a previous study as essential for both catecholamine and secondary conformation functional responses) (Baker et al., 2008) are highlighted in yellow. (B) View of V172, M178, and W182 (highlighted in red) with the key residues for dimerization in TM5 (R205, A206, A210, I218, R229), TM4 (L171), and the proximal region of extracellular loop 2 (W181, R183) identified by Huang et al. (2013) highlighted in purple. Interestingly, V172 and W182 are on the same outward facing surface as the key dimerization residues, with W182 sandwiched between the two residues (W181, R183), and V172 is next to another (L171) identified by Huang et al. (2013).