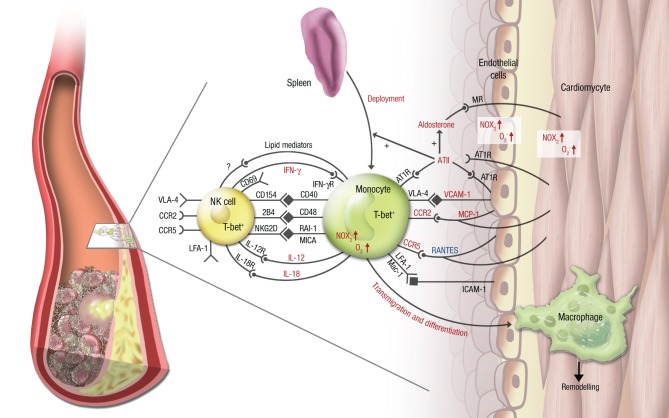
Figure 2.
Vascular inflammation, NK cell/monocyte crosstalk and angiotensin II in cardiac ischemic injury. In cardiac ischemia reperfusion injury and remodeling after MI, vascular inflammation plays a central role. Monocytes transmigrate into the infarcted zone and transdifferentiate into macrophages, that can exert various functions in remodeling depending on the microenvironment provided by cytokines (e.g., IL-12, IL-18, IFN-γ), chemokines (e.g., RANTES/CCL5, MCP-1/CCR2), integrins (e.g., VCAM-1, ICAM-1) and soluble lipid factors like resolvins, lipoxins, and maresins. Signaling through angiotensin II and its downstream hormone aldosterone plays a central mechanistic role in this process. It leads to deployment of AT1R+ monocytes from the spleen to the infarcted area, increases (in red) or attenuates (in blue) chemokine and cytokine expression from the endothelium and from leukocytes, fosters monocyte transmigration and -differentiation and supports a reciprocal program of activation between NK cells and monocytes. While parts of this crosstalk have been partially investigated in this setting (e.g., the T-bet/IFN-γ/IL-12 pathway), most aspects of mutual NK cell/monocyte activation are not known in the setting of vascular inflammation and cardiac ischemic injury. This includes interaction between CD40 and CD154 (Bellora et al., 2010), CD48 and 2B4 (Nedvetzki et al., 2007) and NKG2D and retinoic acid inducible-1 (RAI-1) or members of the MHC class-I related chain (MIC) family of proteins, like MICA (Hamerman et al., 2004; Kloss et al., 2008). All of these are known to increase IFN-γ production by NK cells and CD69 expression on NK cells in infection or lipopoplysaccharide-driven models. Abbreviations: CD, cluster of differentiation; IL, interleukin; ATII, angiotensin II; AT1R, ATII receptor type 1; MR, mineralocorticoid receptor. Red letters: induced/activated by ATII/Aldosterone. Blue letters: attenuated by ATII/Aldosterone.