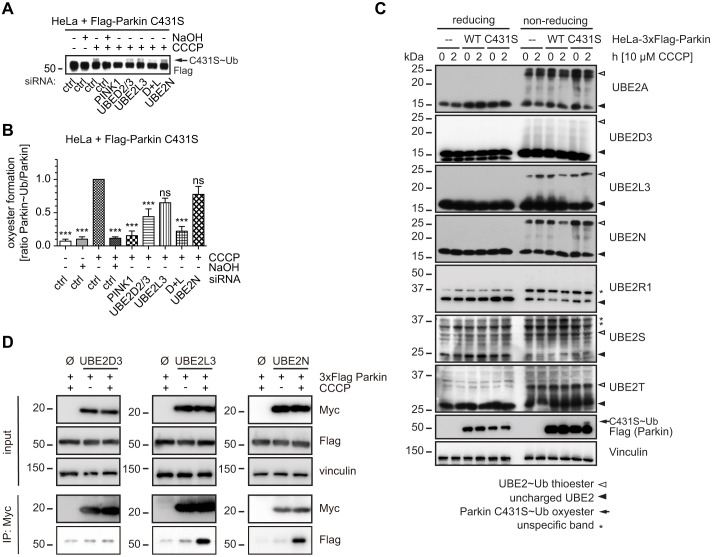
Fig. 7.
UBE2D, UBE2L3 and UBE2N are utilized by Parkin during mitophagy. (A) HeLa cells were transfected with Flag–Parkin C431S upon knockdown of UBE2 enzymes. Cells were treated with CCCP for 4 h and harvested. Western blotting was used to monitor the formation of a Parkin-C431S–ubiquitin oxyester (indicated by an arrow), which appears as a band shift and is sensitive to NaOH treatment. Densitometric analysis of western blots is shown in B. Knockdown of UBE2D2/3 significantly reduced the formation of the Parkin C431S–ubiquitin oxyester. Consistent with the stronger effects of two siRNAs, combined knockdown of UBE2D2/3 and UBE2L3 reduced the formation, similar to siRNA against PINK1. Silencing of UBE2N had no effect on oxyester formation (n≧6, one-way ANOVA, Tukey's post-hoc, P<0.0001, F = 9.911). (C) Parental HeLa cells and HeLa cells that stably overexpressed 3×Flag-tagged Parkin wild type (WT) or C431S were left untreated or were treated with 10 µM CCCP for 2 h. Cells were lysed in low pH MES buffer and subjected to SDS-PAGE under reducing and non-reducing conditions. The resulting western blots were probed with antibodies against endogenous UBE2 enzymes to monitor unmodified UBE2 enzymes (closed arrowheads) and 8-kDa shifted ‘charged’ ubiquitin-bound UBE2 enzymes (open arrowheads). HeLa 3×Flag–Parkin WT cells showed a decrease of ubiquitin ‘charged’ species for UBE2D3, UBE2L3 and UBE2N. By contrast, parental HeLa cells and HeLa cells that expressed non-functional C431S Parkin did not show altered thioester levels upon treatment with CCCP. No obvious changes in this ubiquitin-discharge were found for the other E2 enzymes analyzed. (D) Co-immunoprecipitation of Parkin with UBE2 enzymes after treatment with CCCP. HeLa cells that stably expressed 3×FLAG–Parkin wild type were transfected with Myc-tagged E2 enzymes (Myc–UBE2D3, Myc–UBE2L3, Myc–UBE2N) or an empty vector control (Ø). Cells were left untreated or treated with CCCP for 3 h followed by immunoprecipitation for Myc. Upon CCCP treatment, Parkin specifically and strongly co-immunoprecipitated with Myc–UBE2L3 and Myc–UBE2N. ***P<0.0005; ns, not significant.