Abstract
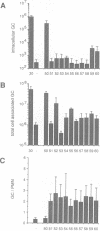
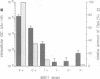
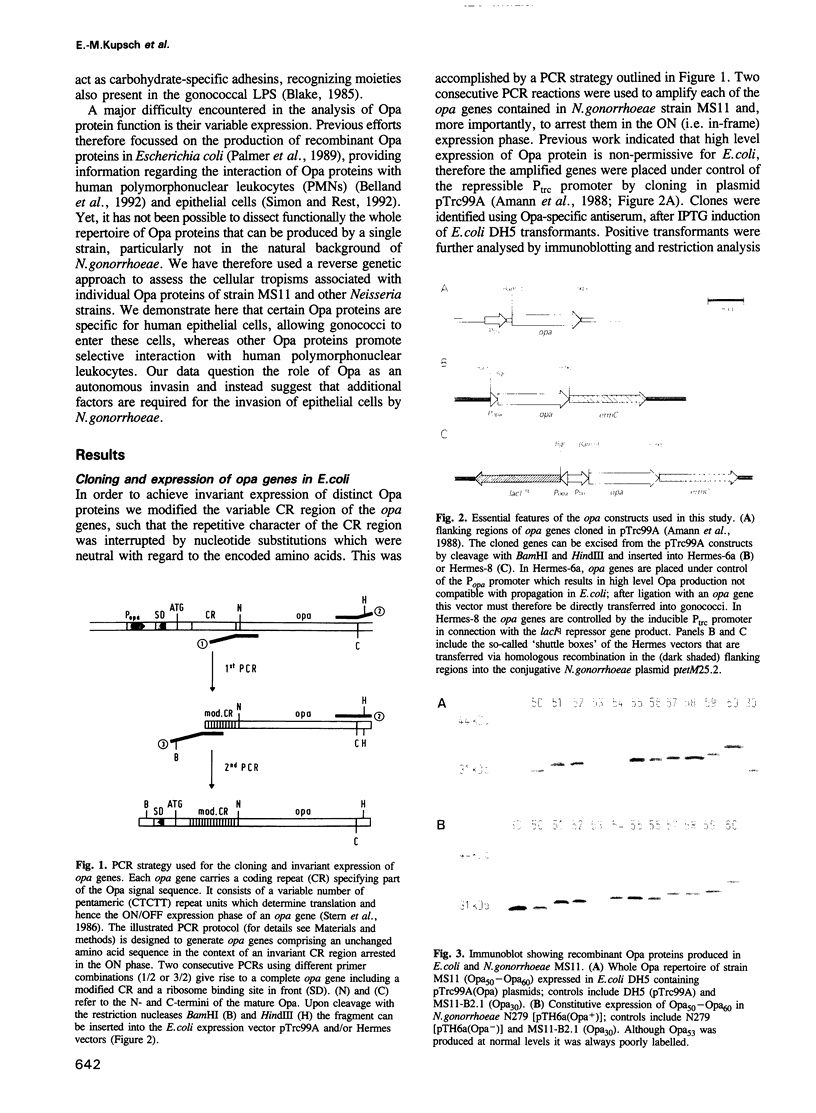
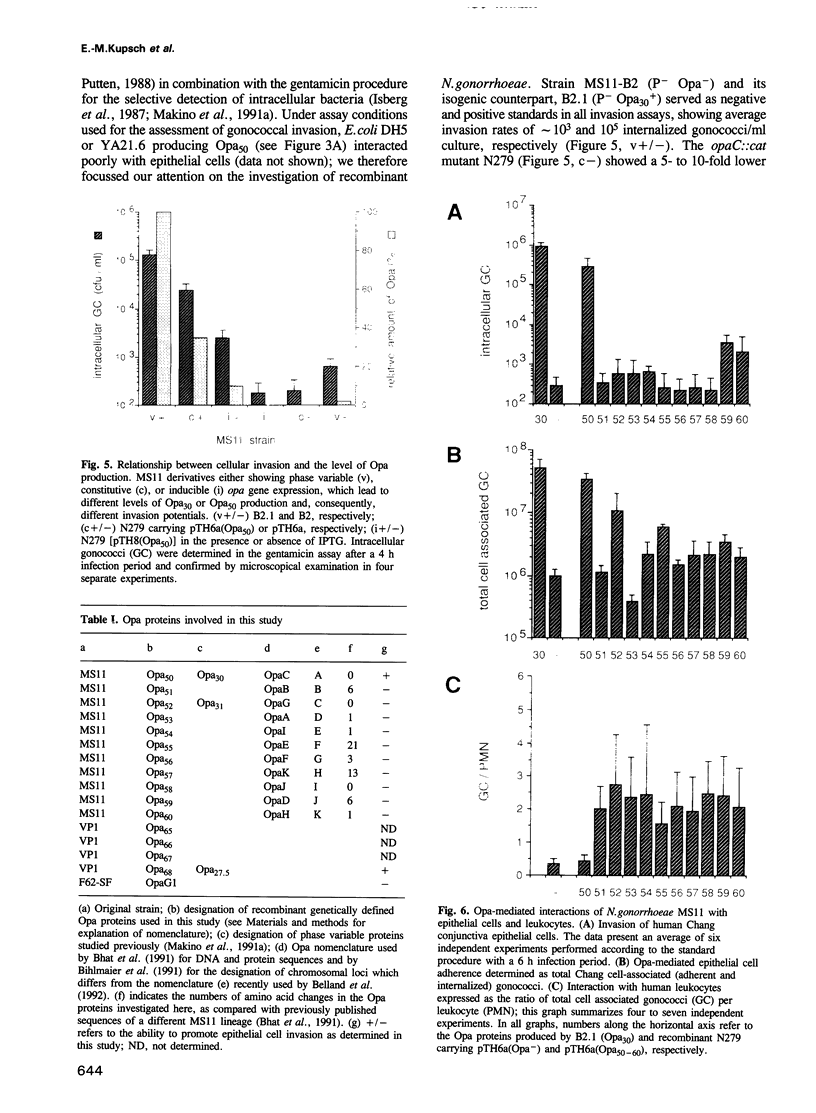
Opacity proteins (Opa) of Neisseria gonorrhoeae, a family of variant outer membrane proteins implicated in pathogenesis, are subject to phase variation. In strain MS11, 11 different opa gene alleles have been identified, the expression of which can be turned on and off independently. Using a reverse genetic approach, we demonstrate that a single Opa protein variant of strain MS11, Opa50, enables gonococci to invade epithelial cells. The remaining variant Opa proteins show no, or very little, specificity for epithelial cells but instead confer interaction with human polymorphonuclear neutrophils (PMNs). Thus, depending on the opa allele expressed, gonococci are capable of invading epithelial cells or of interacting with human leukocytes. The respective properties of Opa proteins are maintained independent of the gonococcal strain; thus, the specificity for epithelial cells or leukocytes is intrinsic to Opa proteins. Significant homology exists in the surface exposed variable regions of two invasion supporting Opa proteins from independent strains. Efficient epithelial cell invasion is favoured by high level Opa production, however, a 10-fold reduction still allows significant invasion by gonococci. In contrast, recombinant Escherichia coli expressing Opa proteins adhered or invaded poorly under similar experimental conditions, thus indicating that additional factors besides Opa are required in the Opa-mediated interaction with human cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
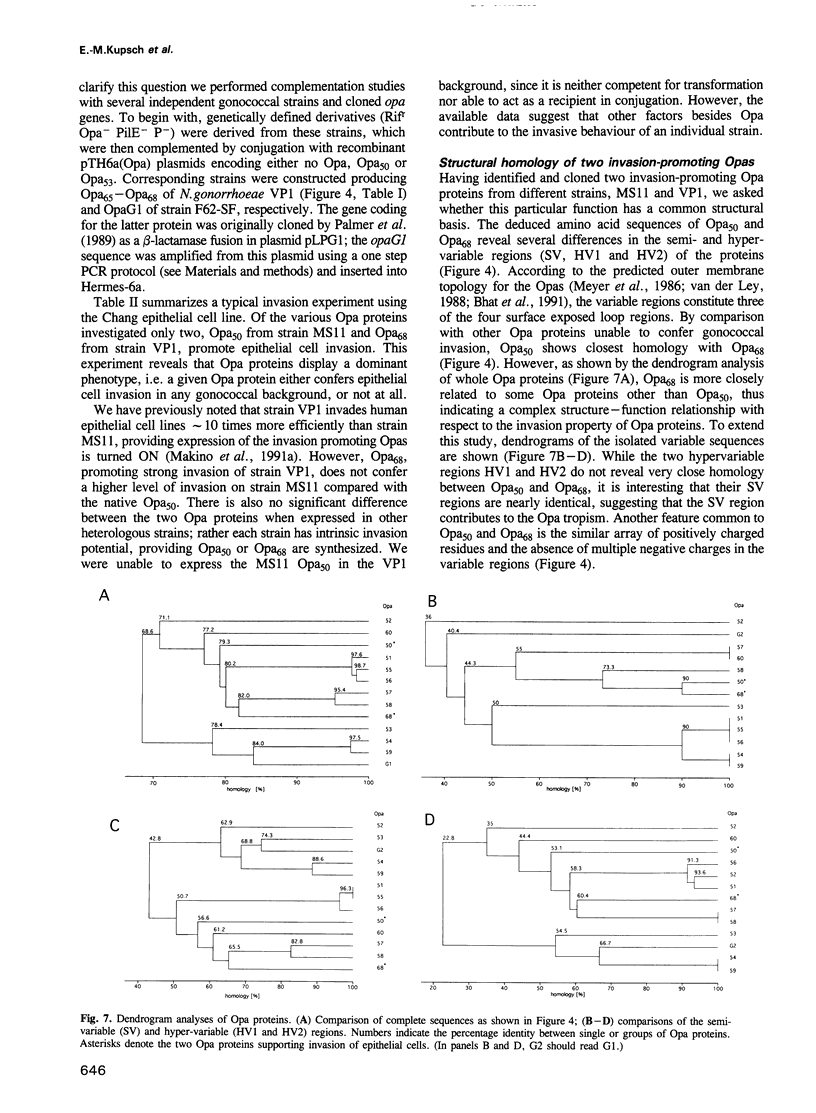
- Amann E., Ochs B., Abel K. J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988 Sep 30;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- Belland R. J., Chen T., Swanson J., Fischer S. H. Human neutrophil response to recombinant neisserial Opa proteins. Mol Microbiol. 1992 Jul;6(13):1729–1737. doi: 10.1111/j.1365-2958.1992.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Belland R. J., Morrison S. G., van der Ley P., Swanson J. Expression and phase variation of gonococcal P.II genes in Escherichia coli involves ribosomal frameshifting and slipped-strand mispairing. Mol Microbiol. 1989 Jun;3(6):777–786. doi: 10.1111/j.1365-2958.1989.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Benkirane R., Guinet R., Delaunay T. Purification and immunological studies of the cross-reaction between the 65-kilodalton gonococcal parietal lectin and an antigen common to a wide range of bacteria. Infect Immun. 1992 Aug;60(8):3468–3471. doi: 10.1128/iai.60.8.3468-3471.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat K. S., Gibbs C. P., Barrera O., Morrison S. G., Jähnig F., Stern A., Kupsch E. M., Meyer T. F., Swanson J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991 Aug;5(8):1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Bhat K. S., Gibbs C. P., Barrera O., Morrison S. G., Jähnig F., Stern A., Kupsch E. M., Meyer T. F., Swanson J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1992 Apr;6(8):1073–1076. doi: 10.1111/j.1365-2958.1992.tb02172.x. [DOI] [PubMed] [Google Scholar]
- Bihimaier A., Römling U., Meyer T. F., Tümmler B., Gibbs C. P. Physical and genetic map of the Neisseria gonorrhoeae strain MS11-N198 chromosome. Mol Microbiol. 1991 Oct;5(10):2529–2539. doi: 10.1111/j.1365-2958.1991.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Chen J. C., Bavoil P., Clark V. L. Enhancement of the invasive ability of Neisseria gonorrhoeae by contact with HecIB, an adenocarcinoma endometrial cell line. Mol Microbiol. 1991 Jun;5(6):1531–1538. doi: 10.1111/j.1365-2958.1991.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Connell T. D., Black W. J., Kawula T. H., Barritt D. S., Dempsey J. A., Kverneland K., Jr, Stephenson A., Schepart B. S., Murphy G. L., Cannon J. G. Recombination among protein II genes of Neisseria gonorrhoeae generates new coding sequences and increases structural variability in the protein II family. Mol Microbiol. 1988 Mar;2(2):227–236. doi: 10.1111/j.1365-2958.1988.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Fischer S. H., Rest R. F. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988 Jun;56(6):1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R., Schwarz H., Meyer T. F. Release of soluble pilin antigen coupled with gene conversion in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9079–9083. doi: 10.1073/pnas.84.24.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Jonsson A. B., Nyberg G., Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991 Feb;10(2):477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Zenilman J. M., Biddle J. W., Perkins G. H., DeWitt W. E., Thomas M. L., Johnson S. R., Morse S. A. Frequency and distribution in the United States of strains of Neisseria gonorrhoeae with plasmid-mediated, high-level resistance to tetracycline. J Infect Dis. 1987 Apr;155(4):819–822. doi: 10.1093/infdis/155.4.819. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Robertson J. N., Watt P. J. Biological properties of two distinct pilus types produced by isogenic variants of Neisseria gonorrhoeae P9. J Bacteriol. 1980 Jan;141(1):393–396. doi: 10.1128/jb.141.1.393-396.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., van Putten J. P., Meyer T. F. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 1991 Jun;10(6):1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E. Further antigenic similarities of Neisseria gonorrhoeae lipooligosaccharides and human glycosphingolipids. Infect Immun. 1992 Jul;60(7):3017–3020. doi: 10.1128/iai.60.7.3017-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981 Mar;143(3):413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Billyard E., Haas R., Storzbach S., So M. Pilus genes of Neisseria gonorrheae: chromosomal organization and DNA sequence. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6110–6114. doi: 10.1073/pnas.81.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Haas R., Stern A., Fiedler H., Frosch M., Jähnig F., Muralidharan K., Veit S. Surface proteins of pathogenic "Neisseria": antigenic variation and conserved epitopes. Ann Sclavo Collana Monogr. 1986;3(1-2):407–414. [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Murphy G. L., Connell T. D., Barritt D. S., Koomey M., Cannon J. G. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989 Feb 24;56(4):539–547. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- Nyberg G., Strömberg N., Jonsson A., Karlsson K. A., Normark S. Erythrocyte gangliosides act as receptors for Neisseria subflava: identification of the Sia-1 adhesin. Infect Immun. 1990 Aug;58(8):2555–2563. doi: 10.1128/iai.58.8.2555-2563.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L., Brooks G. F., Falkow S. Expression of gonococcal protein II in Escherichia coli by translational fusion. Mol Microbiol. 1989 May;3(5):663–671. doi: 10.1111/j.1365-2958.1989.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Kwaasi A. A., Patel P. V., Nairn C. A., Smith H. A determinant of resistance of Neisseria gonorrhoeae to killing by human phagocytes: an outer membrane lipoprotein of about 20 kDa with a high content of glutamic acid. J Gen Microbiol. 1986 Dec;132(12):3277–3287. doi: 10.1099/00221287-132-12-3277. [DOI] [PubMed] [Google Scholar]
- Paruchuri D. K., Seifert H. S., Ajioka R. S., Karlsson K. A., So M. Identification and characterization of a Neisseria gonorrhoeae gene encoding a glycolipid-binding adhesin. Proc Natl Acad Sci U S A. 1990 Jan;87(1):333–337. doi: 10.1073/pnas.87.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Shafer W. M. Interactions of Neisseria gonorrhoeae with human neutrophils. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S83–S91. doi: 10.1128/cmr.2.suppl.s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. D., Meyer T. F. Genetic variation in pathogenic bacteria. Trends Genet. 1992 Dec;8(12):422–427. doi: 10.1016/0168-9525(92)90325-x. [DOI] [PubMed] [Google Scholar]
- Rudel T., van Putten J. P., Gibbs C. P., Haas R., Meyer T. F. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol. 1992 Nov;6(22):3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988 Jun;56(6):1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Rest R. F. Escherichia coli expressing a Neisseria gonorrhoeae opacity-associated outer membrane protein invade human cervical and endometrial epithelial cell lines. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5512–5516. doi: 10.1073/pnas.89.12.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Stern A., Nickel P., Meyer T. F., So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984 Jun;37(2):447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol. 1986 Feb;132(2):503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- Virji M., Kayhty H., Ferguson D. J., Alexandrescu C., Heckels J. E., Moxon E. R. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol Microbiol. 1991 Aug;5(8):1831–1841. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Virji M., Makepeace K., Ferguson D. J., Achtman M., Sarkari J., Moxon E. R. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol Microbiol. 1992 Oct;6(19):2785–2795. doi: 10.1111/j.1365-2958.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- Weel J. F., Hopman C. T., van Putten J. P. In situ expression and localization of Neisseria gonorrhoeae opacity proteins in infected epithelial cells: apparent role of Opa proteins in cellular invasion. J Exp Med. 1991 Jun 1;173(6):1395–1405. doi: 10.1084/jem.173.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weel J. F., van Putten J. P. Ultrastructural localization of gonococcal antigens in infected epithelial cells as visualized by post-embedding immuno-electronmicroscopy. Microb Pathog. 1988 Mar;4(3):213–222. doi: 10.1016/0882-4010(88)90071-x. [DOI] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J Bacteriol. 1982 Aug;151(2):718–722. doi: 10.1128/jb.151.2.718-722.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P. Three copies of a single protein II-encoding sequence in the genome of Neisseria gonorrhoeae JS3: evidence for gene conversion and gene duplication. Mol Microbiol. 1988 Nov;2(6):797–806. doi: 10.1111/j.1365-2958.1988.tb00091.x. [DOI] [PubMed] [Google Scholar]