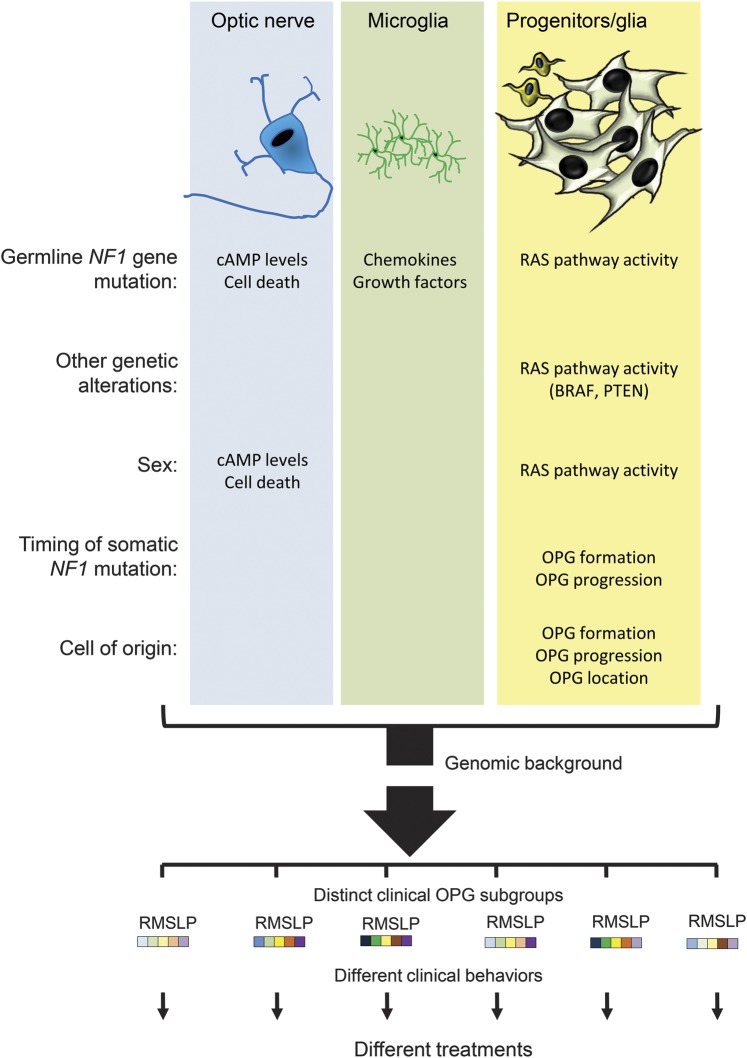
Figure 2. Determinants of NF1-OPG disease heterogeneity.
Neurofibromatosis type 1–optic pathway glioma (NF1-OPG) heterogeneity is determined by a confluence of individual factors that individually affect cell biology and glioma risk. For example, the specific germline NF1 gene mutation creates differential effects on cyclic adenosine monophosphate (cAMP) levels and retinal ganglion cell (RGC) death in neurons (denoted R in the subgroup bar code), chemokine and growth factor production in microglia (denoted M in the subgroup bar code), and RAS pathway activation in neoplastic progenitors/glia (denoted S in the subgroup bar code). Similarly, other genetic alterations (KIAA1549:BRAF or PTEN mutation) alter the activity of the RAS pathway relevant to NF1-deficient tumor cell growth. In addition, patient sex leads to differences in cAMP levels (neurons) or RAS pathway activity (neoplastic progenitors/glia) to affect RGC survival or optic glioma growth. Likewise, the timing of and cell type with somatic NF1 gene inactivation influences NF1-OPG brain location (optic nerve vs postchiasmal tracts, denoted L in the subgroup bar code) or clinical features (clinical progression, denoted P in the subgroup bar code). Finally, the genomic background represents another strong determinant of tumor development and progression. Together, these factors (depicted as colored boxes to illustrate their relative effects) could be used to construct risk assessment algorithms that inform clinical practice.