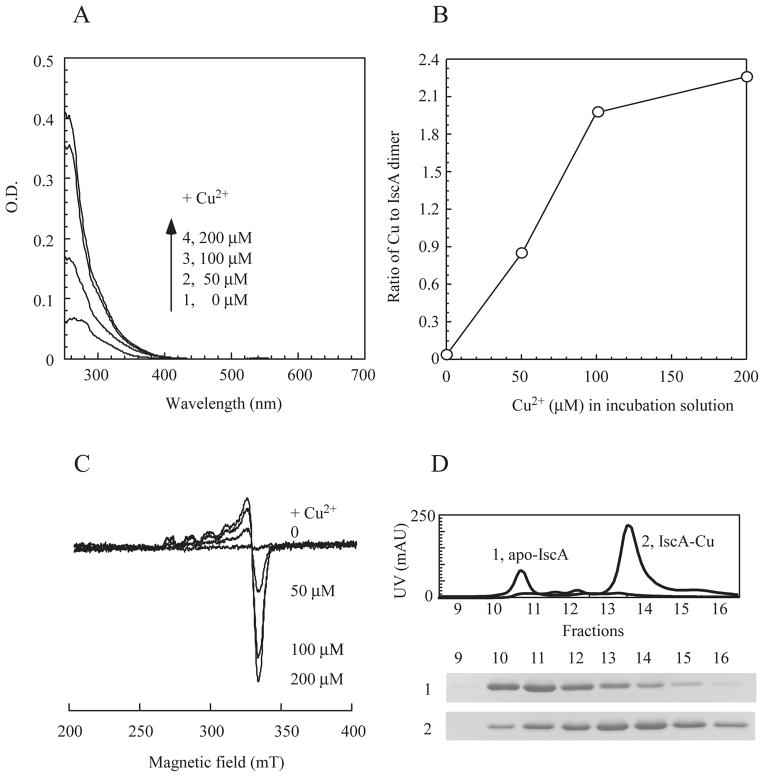
Figure 3. In vitro copper binding activity of IscA.
Apo-IscA (50 μM dimer) was incubated with 0, 50, 100, and 200 μM CuSO4 in the presence of dithiothreitol (2 mM). Protein was re-purified from incubation solutions by passing through a Hi-trap Desalt column. A), UV-visible spectra of re-purified IscA after reconstitution with the indicated concentration of CuSO4. B), relative copper binding activity of IscA. The copper content of re-purified IscA was analyzed and plotted as a function of the CuSO4 concentration in the incubation solution. C), the EPR spectra of the copper-bound IscA. Re-purified IscA proteins were treated with 2.5% (v/v) nitric acid and subjected to the EPR measurements. D), elution profiles of apo-IscA and the copper-bound IscA from a Mono-Q column. The copper-bound IscA was prepared after apo-IscA (50 μM dimer) was incubated with 200 μM CuSO4 in the presence of dithiothreitol (2 mM). Top, elution profiles of apo-IscA (trace 1) or the copper-bound IscA (trace 2) using a linear gradient of NaCl (0 to 0.5 M). Bottom, photographs of the SDS-PAGE gel of the eluted fractions of apo-IscA (sample 1) and the copper-bound IscA (sample 2).