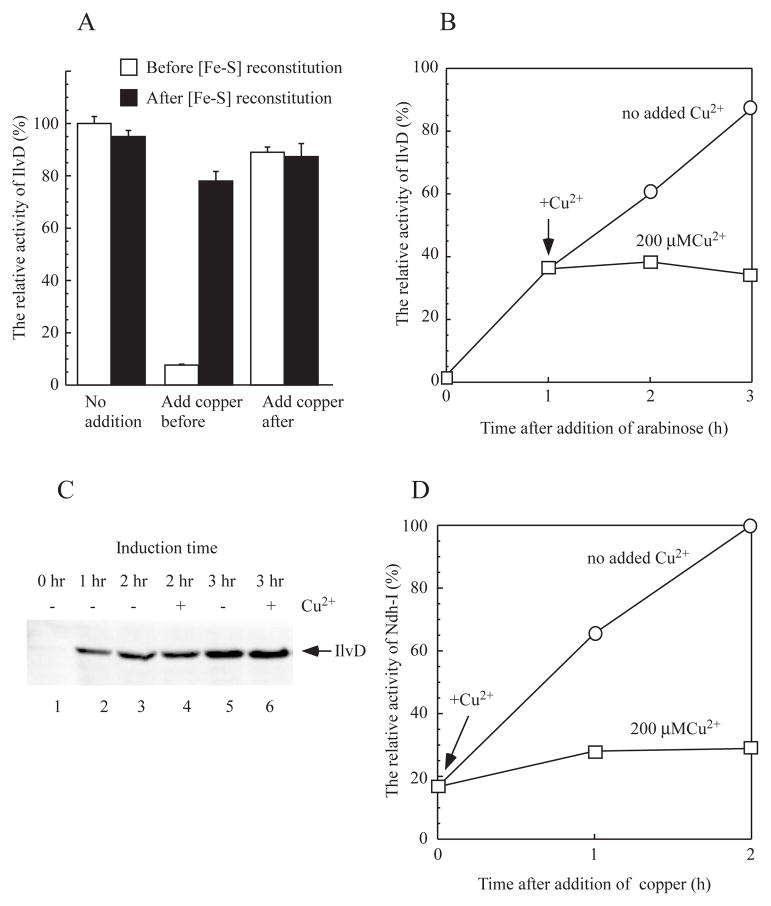
Figure 6. Copper largely blocks the [4Fe-4S] cluster assembly in the E. coli cells.
A), inhibition of the [4Fe-4S] cluster assembly in recombinant dihydroxyacid dehydratase (IlvD) in the E. coli cells by copper. Recombinant IlvD was expressed in the E. coli copA/cueO/cusA mutant cells grown in LB media. CuSO4 (200 μM) was added to the cell culture before or after recombinant IlvD was produced in the cells. Cell extracts were prepared, and the enzyme activity of IlvD in the cell extracts was measured before (open bars) and after (closed bars) the cell extracts were incubated with L-cysteine (1 mM), cysteine desulfurase IscS (1 μM), Fe(NH4)2(SO4)2 (100 μM) and dithiothreitol (2 mM) under anaerobic conditions. B), effect of copper on the IlvD [4Fe-4S] cluster assembly in the E. coli copA/cueO/cusA mutant cells. CuSO4 (200 μM) was added to the cell culture after recombinant IlvD was expressed for one hour. Cell extracts were prepared at indicated time points, and the enzyme activity of IlvD in the cell extracts was measured and plotted as a function of cell growth time. C), effect of copper on the recombinant IlvD expression in the E. coli copA/cueO/cusA mutant cells. The cell extracts were prepared and analyzed by Western blotting using the antibody against His-tag. Lane 1, at time 0; lane 2, after 1 hour induction; lanes 3 and 4, after 2 hours induction; lanes 5 and 6, after 3 hours induction. Lanes 3 and 5, with no copper addition; lanes 4 and 6, with CuSO4 (200 μM). D), effect of copper on NADH dehydrogenase I in the E. coli cells. Overnight cells of the E. coli copA/cueO/cusA mutant were grown for three hours, and treated with or without CuSO4 (200 μM). The enzyme activity of NADH dehydrogenase I in the E. coli cells was measured at 0, 1 and 2 hours after addition of CuSO4. The relative enzyme activity of NADH dehydrogenase I was plotted as a function of cell growth time. The results are the representatives from three independent experiments.