Figure 1.
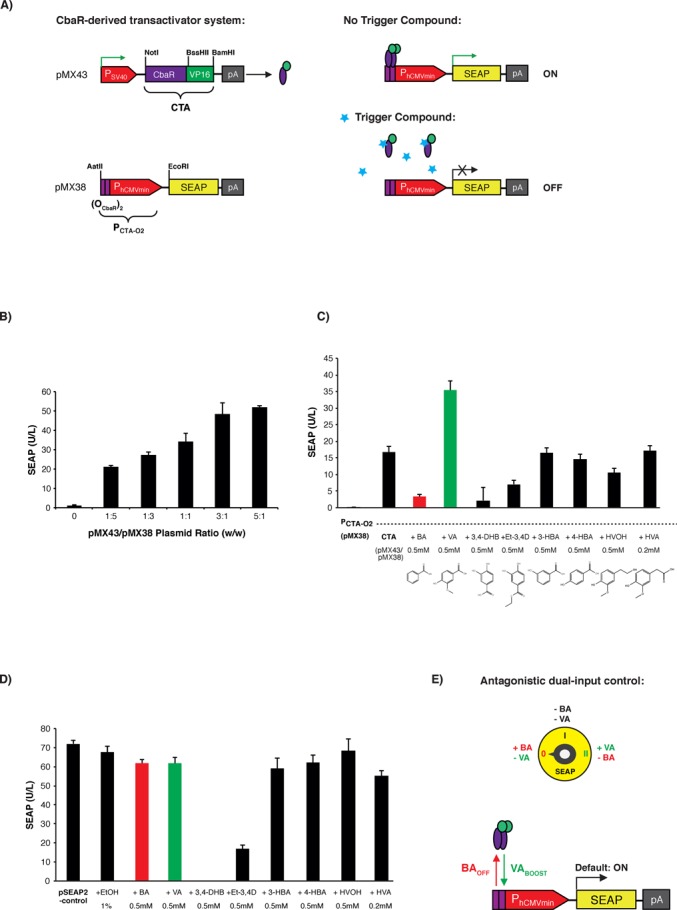
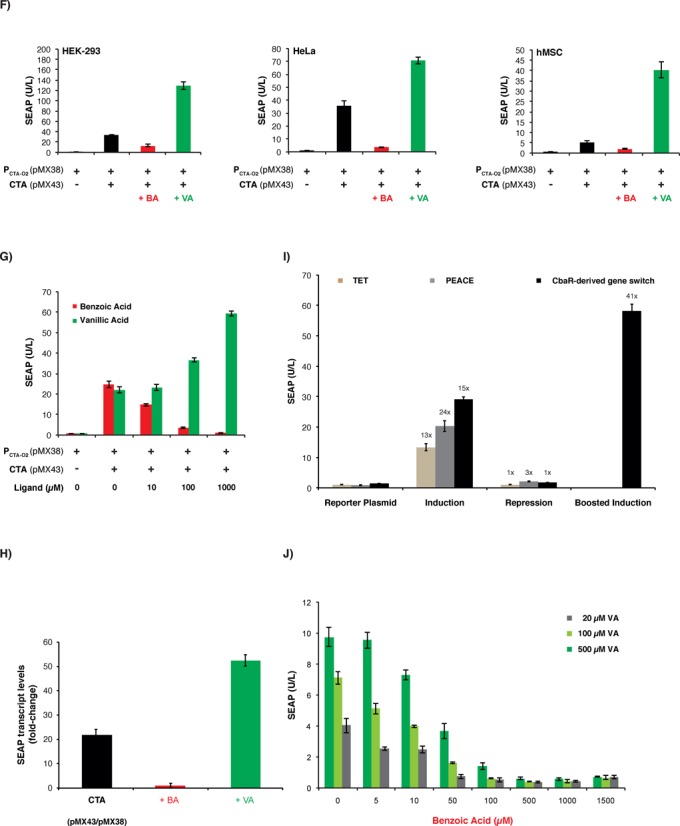
Comamonas testosteroni CbaR-derived transactivation-based mammalian gene switch. (A) Design and functionality of the CbaR-derived transactivation-based transgene control system. C′-terminal fusion of CbaR to the transactivation domain VP16 of the Herpes simplex virus results in a CbaR-derived transactivator CTA (pMX43, PSV40-CTA-pA; CTA, CbaR-VP16). Following constitutive expression by the Simian virus 40 promoter (PSV40), CTA binds and activates a chimeric promoter PCTA-O2 (pMX38, PCTA-O2-SEAP-pA; PCTA-O2, (OCbaR)2-PhCMVmin) containing a tandem CbaR/CTA-specific operator module (OCbaR)2 5′ of a minimal human cytomegalovirus immediate early promoter (PhCMVmin), which is set to drive expression of human placental secreted alkaline phosphatase (SEAP). In the presence of trigger compounds, CTA is released from PCTA-O2, and SEAP expression is shut down. (B) Validation of CTA-mediated PCTA-O2-driven SEAP expression in HEK-293 cells. HEK-293 cells were cotransfected with different ratios (w/w; 0.6 μg total DNA) of transactivator-encoding pMX43 and the SEAP reporter plasmid pMX38, and SEAP expression was scored in the culture supernatant after 48 h. The data are show as the mean ± SD, n = 3. (C) Impact of small-molecule compounds on CTA/PCTA-O2-controlled transgene expression. HEK-293 cells cotransfected with pMX43 and pMX38 at a 1:3 ratio (w/w; 2 μg total DNA) were reseeded at a standard cell density in a 96-well plate (15 000 cells in 100 μl per well) containing potential trigger compounds at the indicated concentrations (BA: benzoic acid; VA: vanillic acid; 3,4-DHB: 3,4-dihydroxybenzoic acid; Et-3,4D: ethyl 3,4-dihydroxybenzoate; 3-HBA: 3-hydroxy-benzoate; 4-HBA: 4-hydroxy-benzoate; HVOH: homo-vanillyl-alcohol; HVA: homo-vanillic-acid). pMX38-transfected cells were used as a basal expression control. The SEAP levels were profiled in the culture supernatant after 48 h. The data are shown as the mean ± SD, n = 3. (D) Cytotoxicity score of the control compounds shown in Figure 1C. Cytotoxicity was assessed by scoring the impact of control molecules on viability and metabolic integrity by profiling SEAP expression of HEK-293 cells transfected with the constitutive SEAP expression vector pSEAP2-control. pSEAP2-control-transfected HEK-293 cells cultivated in the absence of any compound and HEK-293 cells cultivated in the presence of 1% EtOH, which represents the final solvent concentration present in compound-containing cultures, were used as controls. The SEAP levels were profiled in the culture supernatant 48 h after transfection and compound addition. The data are shown as the mean ± SD, n = 3. (E) Schematic of the step switch-like control characteristic produced by the dual-input CbaR-derived gene switch. CTA transactivates PCTA-O2 while accepting both boosting (vanillic acid, VABOOST) and inactivating (benzoic acid, BAOFF) control inputs. These three distinct output levels (OFF, ON, BOOST) are reminiscent of an electrical stepping switch (0, I, II). (F) Dual-input control characteristic of the CTA/PCTA-O2 expression device. HEK-293, HeLa and hMSC cells were cotransfected with pMX43 and pMX38 at a 1:3 ratio (w/w; 0.5 μg total DNA). The SEAP levels were profiled in the culture supernatant 48 h after cultivation in medium containing either benzoic acid (BA, 750 μM) or vanillic acid (VA, 750 μM). Compound-free and pMX38-transfected cultures were used as controls. The data are shown as the mean ± SD, n = 3. (G) Dose-dependent dual-input control of the CTA/PCTA-O2 gene switch. HeLa cells were cotransfected with pMX43 and pMX38 at a 1:3 (w/w; 0.5 μg total DNA) ratio, and SEAP levels were profiled in the culture supernatant 48 h after cultivation of the cells in medium containing different concentrations (0–1000 μM) of either benzoic or vanillic acid. pMX38-transfected cells were used as a basal expression control. The data are shown as the mean ± SD, n = 3. (H) qRT-PCR analysis of SEAP mRNA levels. HeLa cells were cotransfected with pMX43 and pMX38 at a 1:3 ratio (w/w; 2 μg total DNA) and cultivated for 48 h in medium containing either benzoic acid (BA, 750 μM), vanillic acid (VA, 750 μM) or no control compound (CTA). The SEAP transcript levels were profiled relative to the basal expression control (cells transfected only with pMX38). The analysis was performed in triplicate. (I) Comparative analysis of different mammalian small molecule-repressible transcription-control systems. HeLa cells were cotransfected with the TET- (pSAM200/pMF111), PEACE- (pMG11/pMG10) and CbaR-derived (pMX43/pMX38) control components at a 1:1 ratio (w/w; 0.5 μg total DNA) and cultivated for 48 h in the presence (+, repression) or absence (−, induction) of the corresponding regulating (2 μM tetracycline, 40 μM phloretin and 750 μM benzoic acid) and boosting (750 μM vanillic acid) compounds before SEAP was quantified in the culture supernatant. Cells transfected exclusively with the reporter constructs (TET, pMF111; PEACE, pMG10; CbaR-derived gene switch, pMX38) were used as negative controls. The data are shown as the mean ± SD, n = 3. Fold-changes in SEAP expression were calculated by dividing absolute SEAP levels (induction, repression, boosted induction) by the basal SEAP levels produced by the reporter plasmid used as isogenic negative control. (J) Dose-dependent benzoic acid-mediated repression of vanillic acid boosted SEAP expression by the CTA/PCTA-O2 device. HeLa cells were cotransfected with pMX43 and pMX38 at a 1:3 ratio (w/w; 2 μg total DNA), collected in culture medium containing different concentrations of vanillic acid (20, 100 or 500 μM) and reseeded at standard concentration into the wells of a 96-well plate (15 000 cells in 100 μl per well) containing the indicated concentrations of benzoic acid. Forty-eight hours after the addition of benzoic acid, the SEAP levels were profiled in the culture supernatant. The data are shown as the mean ± SD, n = 3.
