Figure 3.
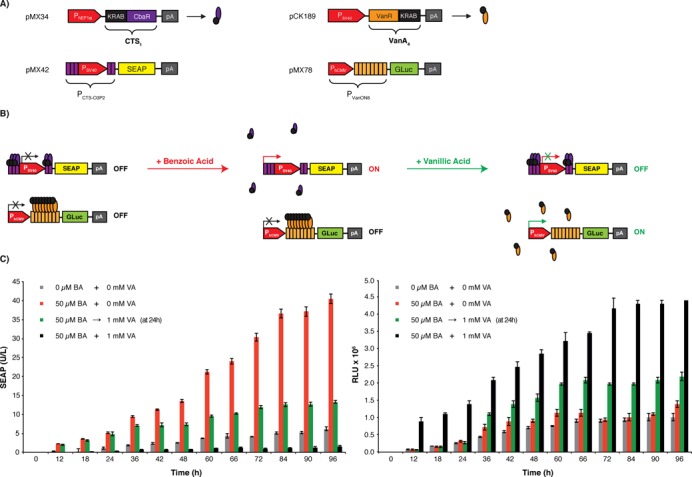
A synthetic gene network with a vanillate control interface. (A) Componentry of the higher-order control network combining two different orthogonal gene switches with antagonistic responses to vanillic acid, the CTS1/PCTS-O3P2 (pMX34, PhEF1α-CTS1-pA; pMX42, PCTS-O3P2 -SEAP-pA) driving SEAP expression and the VACON system (pCK189, PSV40-VanA4-pA; pMX78, PVanON8-GLuc-pA) controlling the expression of GLuc (Gaussia princeps luciferase). The VACON system consists of a vanillic acid-dependent transsilencer VanA4, a fusion of the Caulobacter crescentus repressor VanR and KRAB, which binds and represses PVanON8, a chimeric promoter containing an octameric VanA4-specific operator module (VanO8) 3′ of the human cytomegalovirus immediate early promoter (PhCMV). (B) Operation of the vanillic acid-responsive designer network providing vanillic acid-triggered mutually exclusive expression switches from SEAP to GLuc expression. At the same time as vanillic acid attenuates benzoate-induced CTS1/PCTS-O3P2-driven SEAP expression, it turns on VanA4/PVanON8-driven GLuc expression. (C) Vanillic acid-programmed SEAP and GLuc expression kinetics. HeLa cells were cotransfected with pMX34, pMX42, pCK189 and pMX78 (10:10:1:0.5 ratio [w/w]; 2 μg total DNA) and cultivated for 96 h in the presence or absence of vanillic acid (VA, 1 mM) and benzoic acid (BA, 50 μM). For one experimental configuration, vanillic acid (1 mM) was added after 24 h to a culture containing benzoic acid (50 μM). SEAP as well as GLuc production were profiled every 12 h. The data are shown as the mean ± SD, n = 3.
