Figure 2.
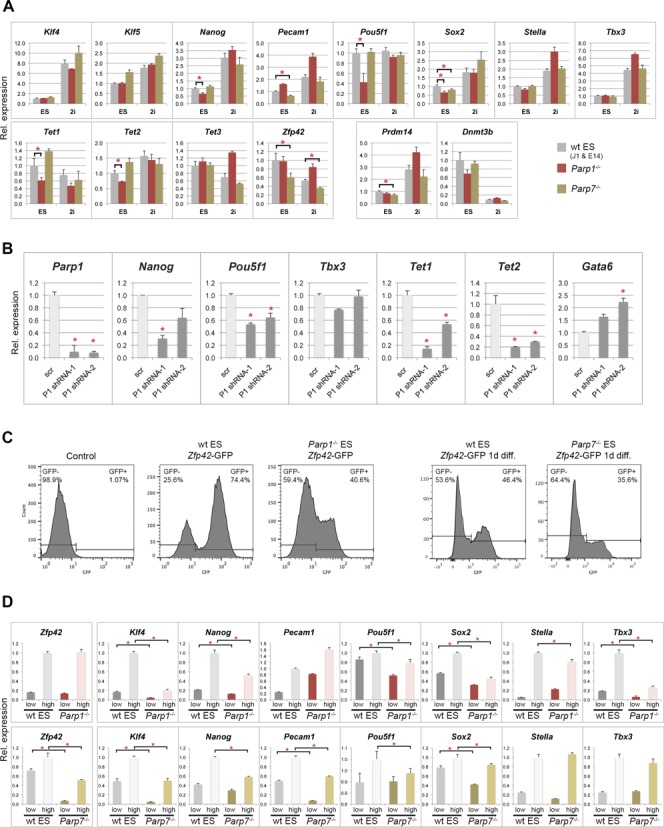
Reduced pluripotency marker expression in Parp1–/– and Parp7–/– ES cells. (A) RT-qPCR analysis of pluripotency markers in wildtype (wt; combined values of J1 and E14 ES cells), Parp1–/– and Parp7–/– ES cells grown in standard ES cell conditions or in ES cell media containing a MEK and GSK3 inhibitor (‘2i’) (*P < 0.05). Prdm14 and Dnmt3b were used as genes particularly responsive to 2i conditions by being up- and down-regulated, respectively (53). (B) Effect of combined deficiency of Parp1 and Parp7, tested in Parp7–/– ES cells transfected with two different shRNA constructs against Parp1 (P1 shRNA-1 and -2) and a scrambled control (scr). Parp1, Pou5f1, Tet1 and Tet2 are significantly down-regulated, and so is Nanog with at least one of the constructs. By contrast, the endoderm differentiation marker Gata6 is up-regulated. (C) Flow cytometric analysis of GFP-negative control ES cells, and wt, Parp1–/– and Parp7–/– ES cells carrying a Zfp42 (= Rex1)-GFPd2 knock-in construct that serves as reporter of Zfp42 expression (38). The proportion of Zfp42-GFP+ cells is significantly reduced in the absence of Parp1 or Parp7. Note that wt and Parp7–/– ES cells analysed after one day of LIF withdrawal (‘1d diff.’) were sorted on a different FACS instrument. In each set the gates were adjusted against the appropriate controls. (D) RT-qPCR analysis of pluripotency gene expression levels in the separated Zfp42-GFP-low and -high cell fractions. Expression levels of most pluripotency markers are reduced, in particular in the Zfp42+/GFP-high fraction, in both Parp1- and Parp7-deficient ES cells.
