FIGURE 3.
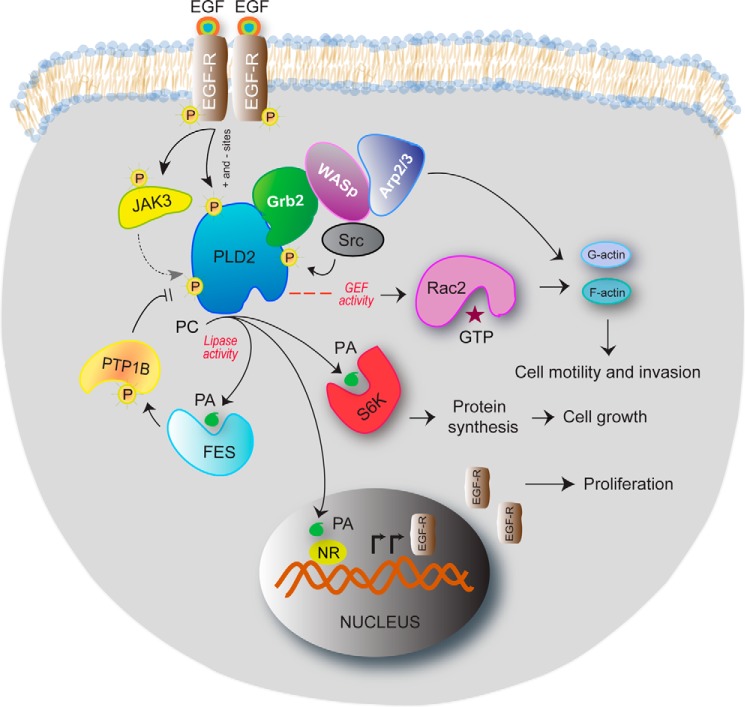
Multiple signaling pathways result in PLD2 contributing to many different steps in this process. As shown in the schematic drawing, PLD2 has many functions in the cell. The invasive phenotype of MDA-MB-231 cells is mediated by PLD2 under control of JAK3 and EGFR. Serum deprivation of cells results in an up-regulated EGFR/JAK3/PLD2-PA system, which is extremely sensitive to JAK3 and PLD2 inhibitors. JAK3 and FES greatly enhance PLD activity following protein-protein interaction through the SH2 domain and the Tyr-415 residue of PLD2. PA enhances FES activity in cancer cells, which provides a positive activation loop between FES and PLD2. PLD2 anchors WASp at the phagocytic cup through Grb2 following protein-protein interactions and also activates it, making key lipids available locally. The heterotrimer PLD2-Grb2-WASp then enables actin nucleation at the phagocytic cup and phagocytosis, which are at the center of the innate immune system function. PLD2 binds to the small GTPase Rac2, which results in a PLD2-GEF activity that switches Rac2 from the GDP-bound to the GTP-bound states, which impacts actin and cell motility. PLD-derived PA binds to ribosomal S6 kinase (S6K), whose enzymatic activity regulates the activation of actin nucleation, and to nuclear receptors (NR) in the nucleus, which contributes to synthesis of EGFR protein and increases cell proliferation.