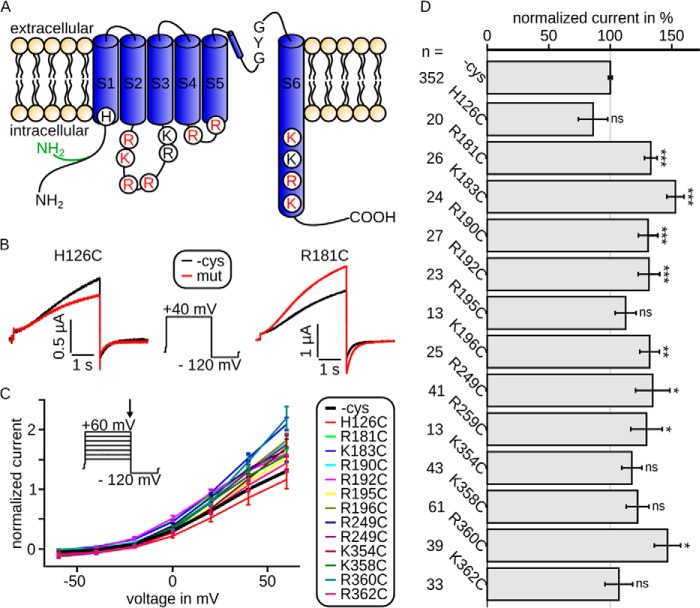
FIGURE 1.
Intracellularly located mutations modulate Kv7.1/KCNE1 channel currents. Currents were recorded 3 days after injection. A, topology model of Kv7.1, showing all six transmembrane segments S1–S6 with pore loop and selectivity filter sequence GYG. Shown are all amino acids included in this study. Red, necessary for interaction with PIP2; black, not relevant for interaction with PIP2 (according to our electrophysiological data; see Fig. 2). The cysteine-free clone used in this study is based on isoform 0, whose N terminus is 95 amino acids shorter than isoform 1 and in which the first 11 amino acids are altered (green). B, representative current traces and voltage protocol with steps from −100 to 40 mV to −120 mV. Current of mutant H126C (red) was slightly decreased compared with wild type (black), whereas the current of mutant R181C was increased. C, mean data ± S.E. of current-voltage relationships between wild type (emphasized) and mutants are similar. The arrow in voltage-step protocol marks the time point of analysis. D, currents at 40 mV were normalized to the corresponding wild type. The mean data ± S.E. (all wild type recordings averaged) show significantly increased (R181C, K183C, R190C, R192C, K196C, R249C, R259C, and R360C) or unchanged (H126C, R195C, K354C, K358C, and K362C) current amplitudes. n, number of oocytes. Asterisks indicate significance: ***, p < 0.001; **, p < 0.01; *, p < 0.05; ns, not significant.