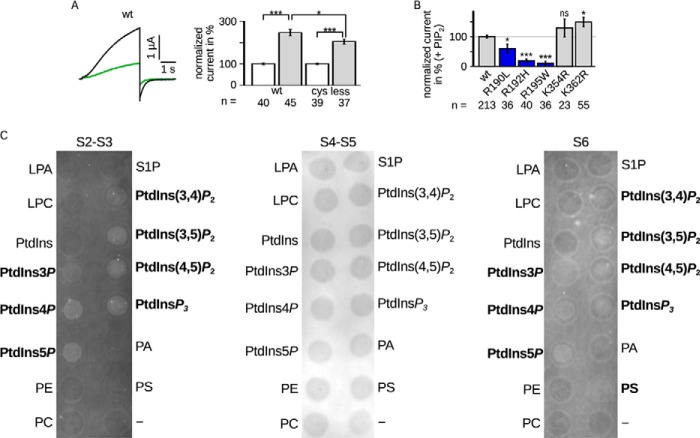
FIGURE 4.
A, LQTS mutants impaired PIP2 regulation. Comparison of Kv7.1 −cys and WT. Left panel, representative current amplitude (black, WT; green, −cys). Right panel, current amplitudes recorded in the presence of diC8-PIP2 were normalized to the corresponding currents without diC8-PIP2. The mean data ± S.E. at 40 mV showed similar changes in currents amplitude. B, LQTS mutants were measured with and without diC8-PIP2. The results with diC8-PIP2 were scaled and normalized to the wild type amplitude of the individual batch of oocytes also with diC8-PIP2. Three mutants were lesser increased (blue). n, number of oocytes. Asterisks indicate significance: ***, p < 0.001; **, p < 0.01; *, p < 0.05; ns, not significant. C, the S2-S3 linker and S6 fragment bound PIP2 in vitro. Representative lipid binding assays of the S2-S3 linker (CRSKYVGLWGRLRFARK), S4-S5 linker (GTWRLLGSVVFIHRQEL), and part of S6 (ALKVQQKQRQKHFNR). Binding of the S2-S3 linker and S6 fragment was demonstrated with PtdIns3P, PtdIns4P, PtdIns5P, PtdIns(3,4)P2, PtdIns(3,5)P2, PtdIns(4,5)P2, and PtdInsP3. S6 also bound to PS. No binding was observed for the S4-S5 linker. Positive results are emphasized. LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; PtdInsP, phosphatidylinositol phosphate; PE, phosphatidylethanolamine; PC, phosphatidylcholine; S1P, sphingosine 1-phosphate; PtdInsP2, phosphatidylinositol bisphosphate; PtdInsP3, phosphatidylinositol trisphosphate; PA, phosphatidic acid; PS, phosphatidyl serine.