FIGURE 1.
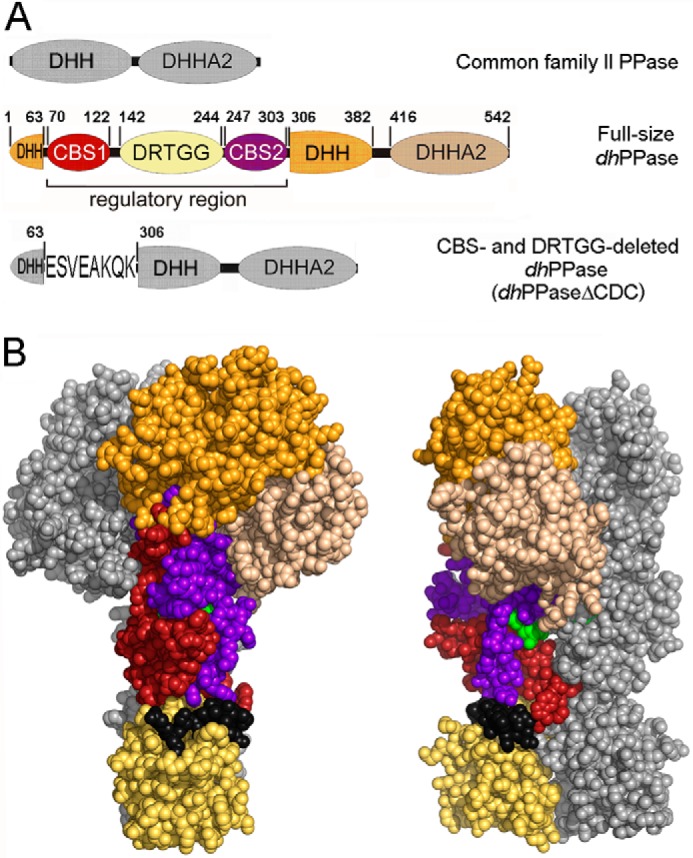
CBS-PPase structure. A, domain arrangement in the primary structures of wild-type dhPPase and its deletion variant. The five domains that form one subunit are shown in different colors and are labeled; short linker regions are depicted in black. DHH and DHHA2 are catalytic domains. Numbers indicate residues that start/end domains or their parts or precede/follow the deleted sequences. Residue numbering is based on the sequence of the full-length protein. For dhPPaseΔCDC, the linker sequence is shown using single letter notation. B, two views of the modeled three-dimensional structure of the cpCBS-PPase homodimers (19) in a sphere representation. Domain colors of one subunit are the same as in A; the other subunit is colored gray. The AMP molecules bound between CBS domains are shown in green.
