FIGURE 5.
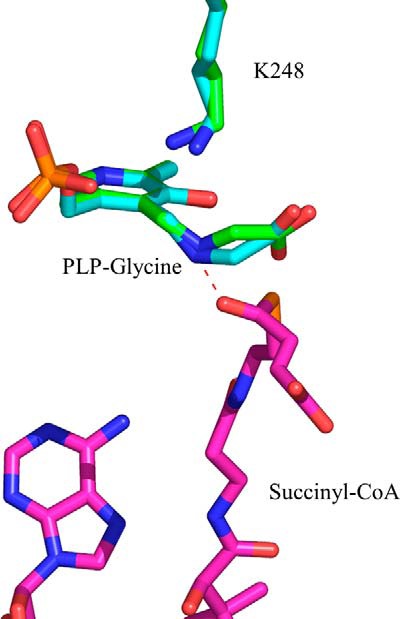
Positioning of the glycine external aldimine in the active site of ALAS. Differences in the positioning of the α-carbon of glycine were detected upon superimposition of the crystal structures for monomers A and E from PDB file 2BWP. The ternary complex formed by the glycine external aldimine and succinyl-CoA was modeled in the active site of ALAS. The succinyl-CoA carbonyl group can strongly interact with the Schiff base nitrogen. The model was built by superimposing the structures with PDB coordinates 2BWP and 2BWO using PyMOL. Amino acid Lys-248 in R. capsulatus ALAS corresponds to Lys-313 in mALAS2.
