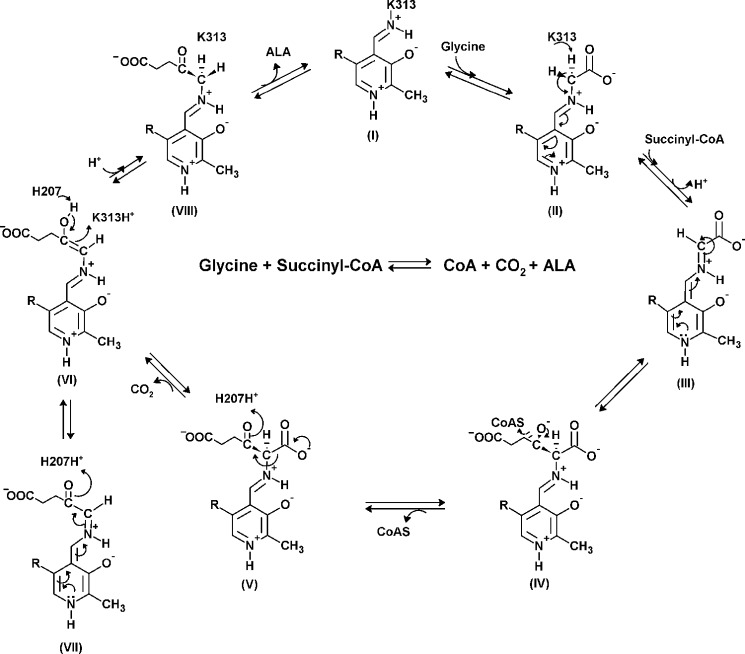
SCHEME 1.
Proposed chemical mechanism for the reaction catalyzed by ALAS. In the resting state, PLP binds covalently to an active site lysine as an internal aldimine (I) (PLP-K313 internal aldimine in mALAS2). The entry of glycine in the active site proceeds with the formation of an external aldimine (II). Removal of the pro-R proton of glycine results in formation of a first quinonoid intermediate (III), which facilitates the condensation with succinyl-CoA and subsequent release of the CoA moiety (IV). When the resulting 2-amino-3-ketoadipate intermediate (V) is decarboxylated (H207-assisted decarboxylation in the mALAS2 reaction), an enol intermediate is formed (VI), which is in rapid equilibrium with a second quinonoid intermediate (VII). The final reaction intermediate is the ALA external aldimine (VIII).