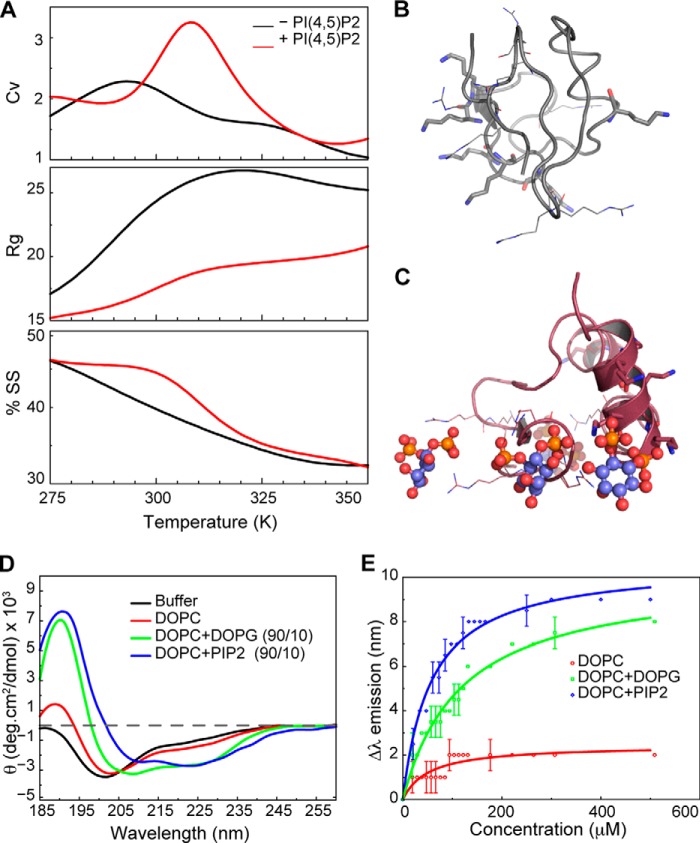
FIGURE 1.
γNT gains secondary structure in the presence of PIP2. A, comparison of structural/thermodynamic parameters of γNT in the presence and absence of PIP2. ENaC γNT is more stable (peak in Cv) and compact (radius of gyration (Rg)) and gains secondary structure (SS) in the presence of PIP2 (red versus black profile). Trajectories from all simulations were used to obtain the reported parameters (“Experimental Procedures”). B, representative structural model of γNT in the absence of PIP2. Lysines and arginines are shown as sticks and lines, respectively. Note that the NT does not present any major secondary structural features. C, representative structural model of γNT in the presence of PIP2. Lysines and arginines are represented as sticks and lines, respectively. PIP2 head groups are shown in ball and stick representation. Notice the preference for helical content in the representative model in the presence of PIP2. D, circular dichroism spectroscopy of γNT in DOPC lipid vesicles doped with DOPG or PIP2 to demonstrate the preference of γNT toward the presence of PIP2 over DOPG to form secondary structures. E, fluorescence anisotropy measurements of γNT peptide in the presence and absence of anioinic phospholipids. In the presence of anionic phospholipids, γNT undergoes a significant secondary structural rearrangement as shown by the emission profile of the NT peptide.