FIGURE 7.
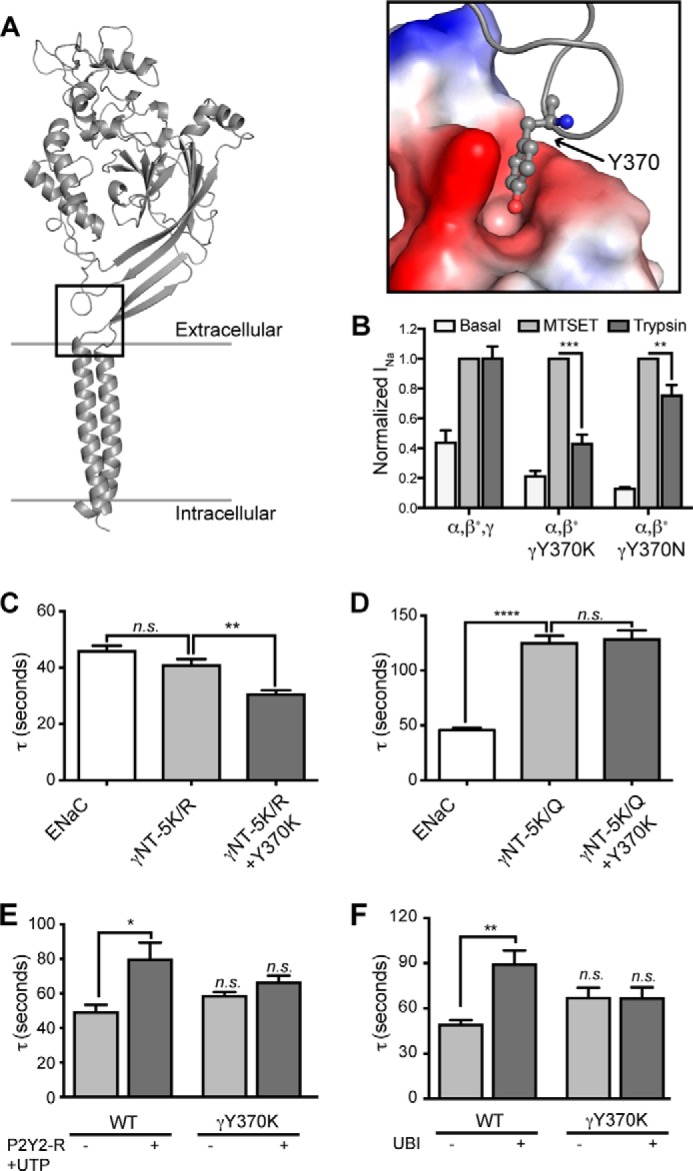
Tyr370 in γENaC mediates allosteric signal propagation required for channel activation in response to cytosolic signaling. A, structural model of rat γENaC constructed based on the crystal structure of cASIC1 (“Experimental Procedures”). Inset, surface representation of the residues in the pocket that forms a docking site for Tyr370. B, oocytes were injected with WT α- and γ-subunits and β-ENaC S518C, resulting in channels that are “locked” in the open state when exposed to the cysteine reactive MTSET. Other oocytes were injected identically except that γ-ENaC Y370K or Y370N replaced WT γ-ENaC. After basal current was recorded, oocytes were exposed to either MTSET (1 mm) or trypsin (20 μg/ml) for 5 min in the presence of amiloride. Amiloride was washed off to reveal the effect of MTSET or trypsin on INa. β-ENaC S518C is indicated by an asterisk on β in each group. C and D, oocytes were injected with WT subunits (ENaC) or with WT α-ENaC and β-ENaC and the indicated mutations of γ-ENaC. Tau was calculated as in Fig. 2. E, WT ENaC coexpressed with P2Y2-R and incubated for 5–20 min with 1 mm UTP were more slowly activated by perfusion with 4 mg/ml chymotrypsin (*, p < 0.05; one-way analysis of variance). UTP did not affect the chymotrypsin activation time constant of INa of ENaC containing γ-ENaCY370K and coexpressed P2Y2-R. F, Xenopus oocytes were injected with WT α- and β-ENaC subunits and either WT (ENaC) or Y370K γ-ENaC (γ370K) (0.3 ng/subunit/oocyte). Oocytes in each group were also injected with ubiquitin (1.0 ng/oocyte) (ENaC + UBI and γY370K + UBI). INa was recorded following washout of amiloride and during 5 min of perfusion with 4 mg/ml chymotrypsin, and the time constant of activation (τ) was calculated as described from four to nine traces performed for each group. **, different from ENaC, p < 0.01 by one-way analysis of variance. n.s., not significant.
