FIGURE 2.
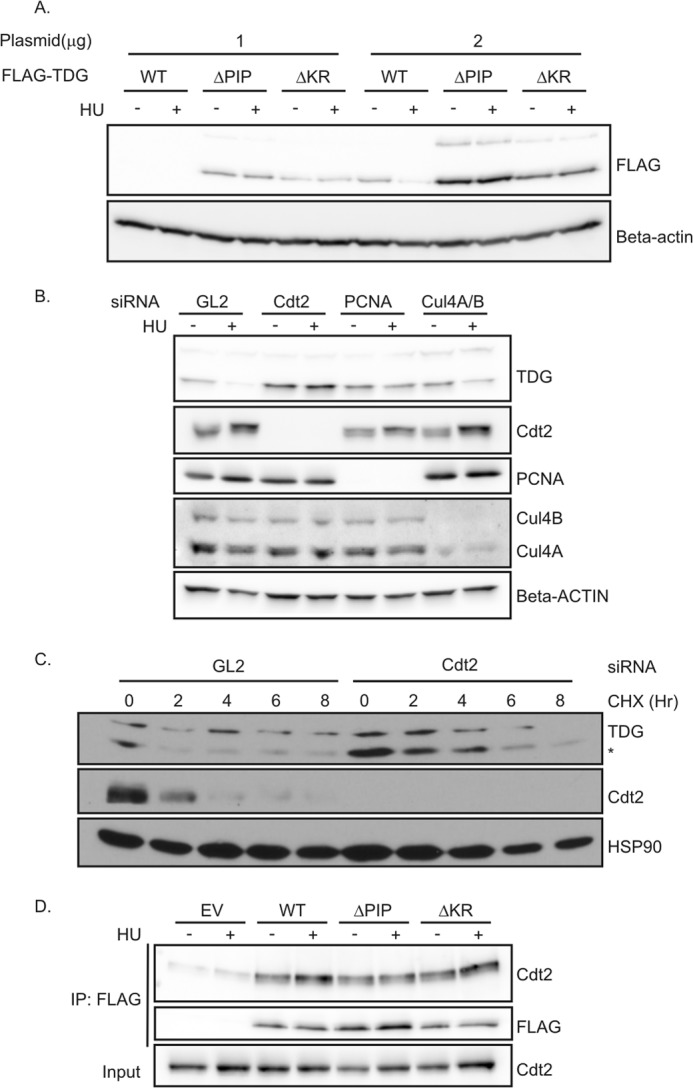
CRL4Cdt2 is required for the degradation of TDG. A, TDG mutants were not degraded after HU treatment. 293T cells in 6-well plates were transiently transfected with 1 or 2 μg of TDG(WT)-, TDG(ΔPIP)-, or TDG(ΔKR)-expressing plasmids for 24 h, followed by treatment with 1.5 mm HU for 24 h. Western blot analyses were performed using the indicated antibodies. B, HeLa cells were treated with the indicated siRNA. siRNA against luciferase (GL2) was used as a negative control. After 48 h, 1.5 mm HU was added for 24 h. Cell lysates were subjected to immunoblotting with anti-TDG and anti-β-actin antibodies. C, knockdown of Cdt2 increased the half-life of TDG. HeLa cells were treated with GL2 siRNA or siRNA against Cdt2 for 60 h, followed by protein synthesis inhibitor cycloheximide treatment (100 μg/ml) for the indicated times. The whole cell lysate was probed with antibodies against indicated proteins. *, lower band is TDG. D, TDG(WT) and mutants co-immunoprecipitate with Cdt2. Nuclear fraction of 293T cells transiently expressing the indicated forms of FLAG-TDG were immunoprecipitated (IP) with anti-FLAG. Association was analyzed by Western blot using anti-Cdt2 and anti-FLAG antibodies. EV, empty vector.
