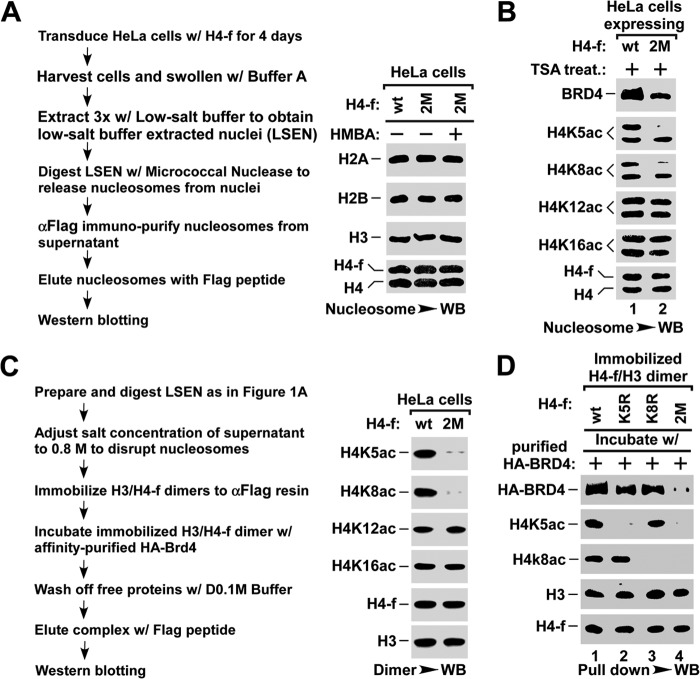
FIGURE 2.
BRD4 binds to nucleosome via H4K5ac/K8ac sites in vivo. A, outline for preparing nucleosome (left panel). Nucleosomes were affinity purified from HeLa cells infected with lentivirus expressing FLAG-tagged wild-type (wt) or K5R/K8R-mutant (2M) H4 using anti-FLAG resin, and analyzed by Western blot (WB) for core histones (right panel). B, the association of BRD4 with nucleosomes containing wt- or K5R/K8R 2M-H4. The nucleosomes were affinity purified from HeLa cells with the indicated lentiviral infection and treatment, and were assayed for the associated BRD4 and the histone modifications by Western blot. C, outline for H3/H4 dimer pulldown assay (left panel) and Western blot analysis of the indicated acetyl-lysine of histone H4 (right panel). D, pulldown assay for the binding of BRD4 to H3/H4 dimers containing wt-, K5R-, K8R-, or K5R/K8R 2M-H4. The H3/H4 dimers containing the indicated H4-f were immobilized onto anti-FLAG resin and incubated with purified HA-BRD4. The amounts of BRD4 bound to H3/H4 dimers were assayed by Western blot.