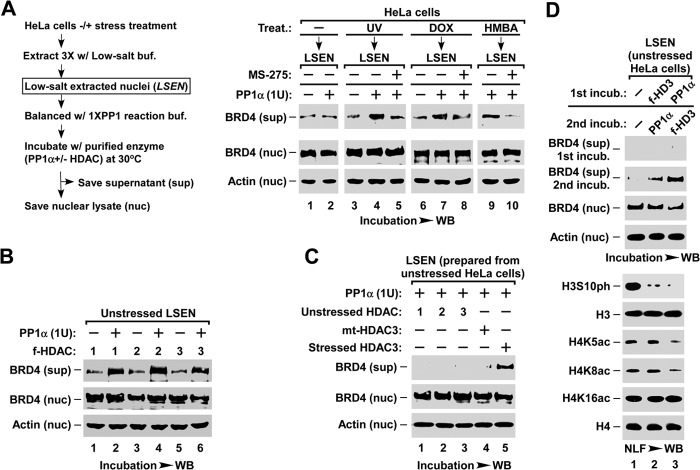
FIGURE 5.
Stress-activated HDAC1/2/3 and PP1α act cooperatively to induce BRD4 release. A, in vitro assay of PP1α-induced BRD4 release in LSEN. LSEN prepared from stressed or unstressed HeLa cells was preincubated with HDAC1/2/3 inhibitor MS-275, followed by incubation with recombinant PP1α enzyme as indicated. For A–D, the levels of BRD4 released into supernatant (sup) or those remaining in chromatin (nuc) were analyzed by Western blot (WB). B, in vitro assay of BRD4 release in LSEN induced by PP1α and HDAC co-incubation. LSEN prepared from unstressed HeLa cells was co-incubated with the indicated enzymes. FLAG-tagged HDAC1, -2, and -3 were affinity purified from HMBA-treated HEK293T cells that had been transfected with the corresponding cDNA constructs, and analyzed by Western blot (right). C, co-incubation with PP1α and non-activated HDAC1, -2, or -3 is unable to induce BRD4 release from the LSEN of the unstressed HeLa cells. For lanes 1-3, the FLAG-tagged wt-HDAC1, -2, and -3 were isolated from unstressed HEK293T cells with corresponding transfections. For lanes 4 and 5, FLAG-tagged mt-HDAC3 and wt-HDAC3 were isolated from stressed HEK293T cells transfected with the corresponding constructs. D, two-round enzymatic incubation assay of LSEN. LSEN of unstressed cells was sequentially incubated with HDAC3 and PP1α enzymes as indicated, with extensive washes between the two incubations. The changes in histone modifications were analyzed by Western blot.