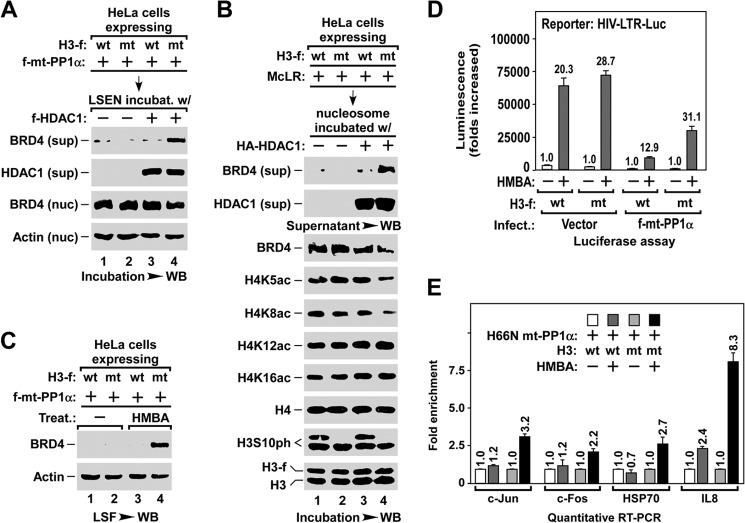
FIGURE 6.
Dephosphorylation of H3S10ph is a prerequisite for stress-induced BRD4 release for inducible gene expression. A, in vitro assay of HDAC1-induced BRD4 release in the LSEN containing either wt- or S10A mt-H3. LSEN of HeLa cells with the indicated lentiviral infection were incubated with HDAC1 enzyme, and the BRD4 released into supernatant (sup) and remained in nuclei (nuc) was analyzed by Western blot (WB). B, in vitro assay of HDAC1-induced BRD4 release from nucleosomes containing either wt- or S10A mt-H3. Nucleosome from HeLa cells with the indicated lentiviral infection and treatment was immobilized on anti-FLAG resin, and incubated with HA-HDAC1 enzyme. The levels of BRD4 released in the supernatant (sup) and remained in nucleosome, and the changes in histone modifications, were analyzed by Western blot. C, HMBA-induced BRD4 release in the cells expressing either wt- or S10A mt-H3. LSF prepared from HeLa cells with the indicated lentiviral infection and HMBA treatment was analyzed by Western blot. D and E, S10A mt-H3 allowing cells to bypass the requirement for PP1α during HMBA-induced expression of inducible genes. HIV-LTR-Luc cells with the indicated lentiviral infection and HMBA treatment were subjected to luciferase assay (D) and qRT-PCR analysis (E).