FIGURE 3.
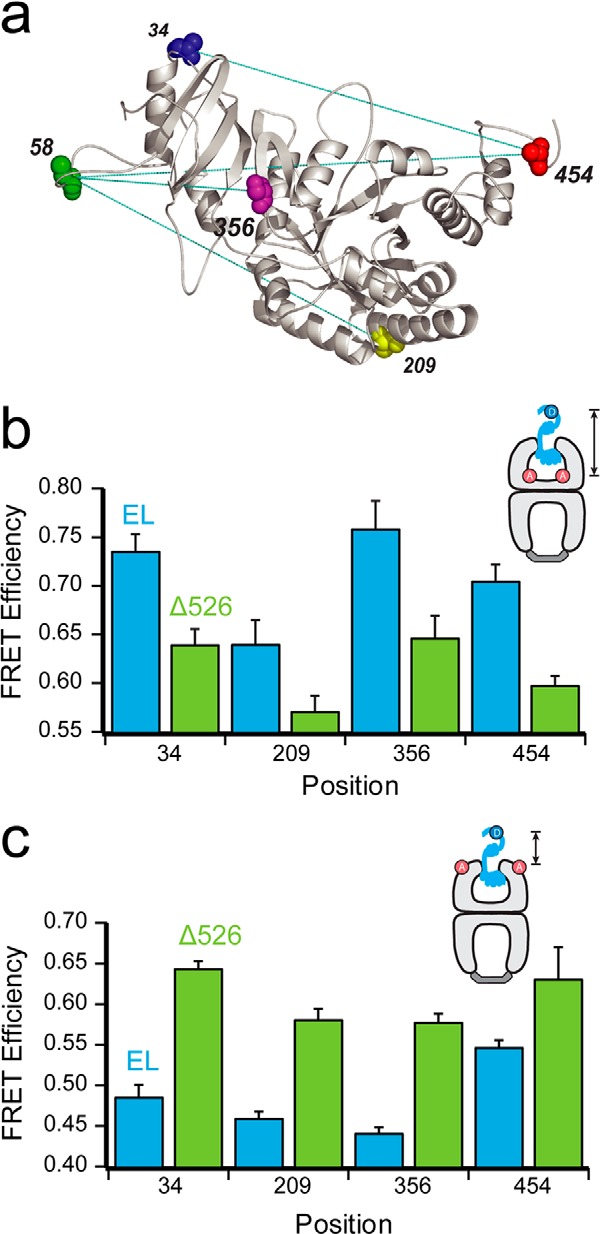
Contact with the C termini promotes deeper initial Rubisco binding within the GroEL cavity. a, structure of one monomer of the native Rubisco dimer (PDB ID: 9RUB) is shown. Positions of five amino acid locations employed for the attachment of exogenous fluorescent probes are indicated. Except for position 58, which is a naturally occurring surface-exposed Cys residue, these positions were mutated to encode Cys and labeled with thiol-alkylating fluorescent dyes as described previously (20, 47). Sites successfully paired for intramolecular FRET assays, in which donor and acceptor dyes are attached to the same Rubisco monomer, are indicated by dotted lines. b, steady-state FRET measurements between chemically denatured, donor-labeled Rubisco (100 nm) bound to the trans ring of acceptor-labeled, full-length (EL; blue) or C-terminal deletion (Δ526; green) GroEL-GroES ADP bullets (120 nm). For these measurements, Rubisco was labeled with EDANS at each of four sites (amino acids 34, 209, 356, and 454), and GroEL was labeled with fluorescein near the bottom of the cavity through a unique, introduced Cys at position 43 (65). Error bars represent the standard deviation for n = 3 experiments. c, steady-state FRET measurements as in b, but using GroEL labeled with fluorescein near the top of the cavity, through a unique introduced Cys at position 315 (20).
