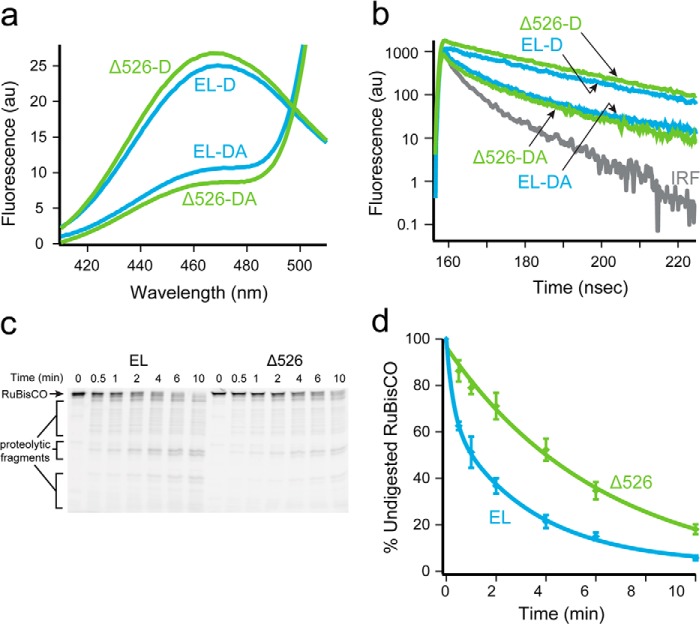
FIGURE 4.
Rubisco adopts a more unfolded conformation on a GroEL ring in the presence of the C termini. a, steady-state fluorescence of chemically denatured, donor-only (209ED) or donor-acceptor (209ED-58F) labeled Rubisco (100 nm) bound to the trans ring of full-length (EL, blue) or C-terminal deletion (Δ526; green) GroEL-GroES ADP bullets (120 nm). The spectra shown are the average of n = 3 experiments. b, time-resolved fluorescence decay measurements of the same complexes in a. The instrument response function is also shown (IRF). c, fluorescently labeled Rubisco (58-F; 100 nm) was chemically denatured and bound to the trans ring of either GroEL or Δ526 ADP bullets (120 nm) and then treated with trypsin for the indicated times before quenching with PMSF (1 mm). The samples were analyzed by SDS-PAGE and laser-excited fluorescence gel scanning. An arrow shows the migration position of full-length Rubisco, and brackets indicate the position of three dominant groups of proteolytic fragments. d, amount of full-length Rubisco remaining at each time point in c was quantified and plotted as a function of time. The average half-time for the digestion of Rubisco bound to full-length ADP bullets was ∼1.5 min (EL, blue) and ∼4 min for C-terminal deletion ADP bullets (Δ526, green). AU, arbitrary units. Error bars represent the standard deviation of n = 3 experiments.