FIGURE 8.
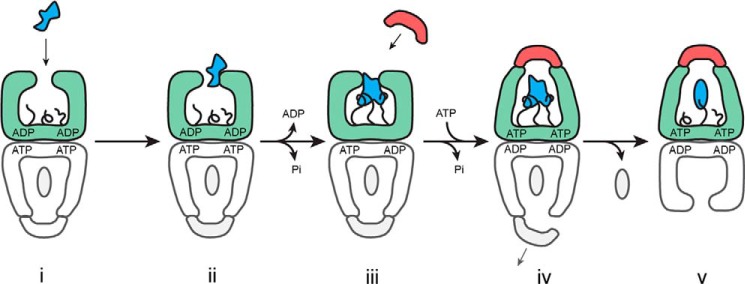
Model for the role of the GroEL C termini in substrate protein unfolding. A schematic is shown for the steps involved in substrate protein loading and the initiation of folding by GroEL. Step i, a non-native substrate protein (irregular blue shape) enters the GroEL reaction cycle on the open trans ring (green) of the ATP bullet complex (21). Step ii, substrate protein binding accelerates both the release of ADP from the trans ring and ATP hydrolysis in the opposite, cis ring (gray) (22, 28, 56, 66). Step iii, binding of the non-native substrate protein by the C-terminal tails (black), helps pull the substrate protein into the GroEL cavity and, in combination with additional binding by multiple apical domains, results in substrate protein unfolding. Step iv, ongoing association of the C termini with the substrate protein during ATP-driven encapsulation by GroES both retards pre-mature protein release (46) and provides an anchor point for forced expansion of the substrate protein as the apical domains shift to accommodate GroES binding. Assembly of the new folding cavity on the trans ring is directly coupled to the disassembly of the folding cavity on the opposite ring, potentially through a transient, symmetric intermediate (28, 53–55). Step v, a subsequent allosteric shift of the GroEL-GroES complex results in full ejection of the substrate protein in the enclosed GroEL-GroES cavity and the initiation of folding (46). The C-terminal tails may continue to interact with the folding intermediate, influencing the spectrum of states populated during folding.
