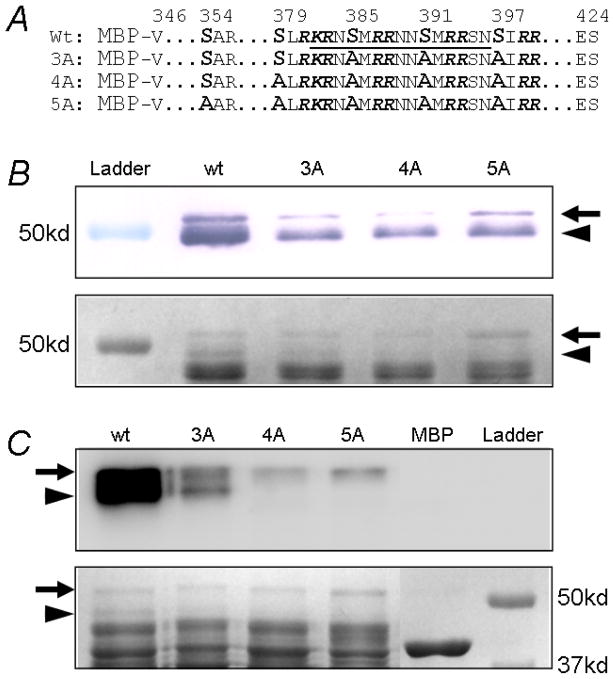
Figure 6.
In vitro phosphorylation on MBP-fusion proteins. A. Four MBP-fusion proteins were constructed by linking Kir6.1 distal C fragment (residues 346–424) to MBP with or without mutations on critical serine residues. The sequence underlined is the antigen for Western detection. B. All the constructs showed two bands with Western blot. The band around 52kd represented the intact fusion proteins (arrow), while the band of ~49kd was a degraded protein fragment (arrowhead). These bands were weekly detected in 3A, 4A and 5A by Western because mutations were made in antigen region. Low panel showed protein input colored with Coomassie blue. C. In vitro phosphorylation showed strong 32P incorporation in both 52kd and 49kd bands in wt. The 32P incorporation was marked reduced in 3A. The 4A and 5A were only weekly phosphorylated in the 52kd band. By comparing 3A and 4A, the phosphorylation of 49kd band on 3A must occur on Ser379. The 49kd of 4A was not phosphorylated also suggested that Ser354 was not phosphorylated. In addition, phosphorylation on the 52kd of 4A and 5A suggested there are unidentified phosphorylation site(s) located C-terminal to Ser397, but they were not functionally important according to mutational analysis in Figure 5E.