Abstract
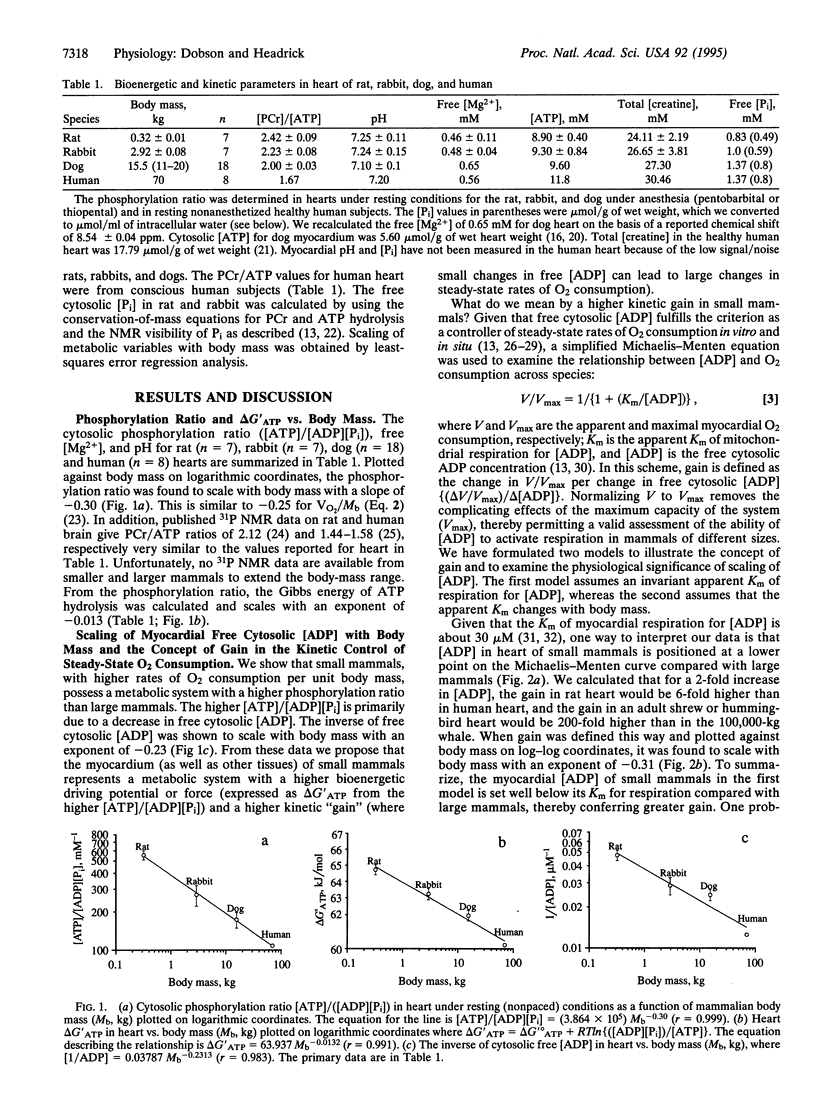
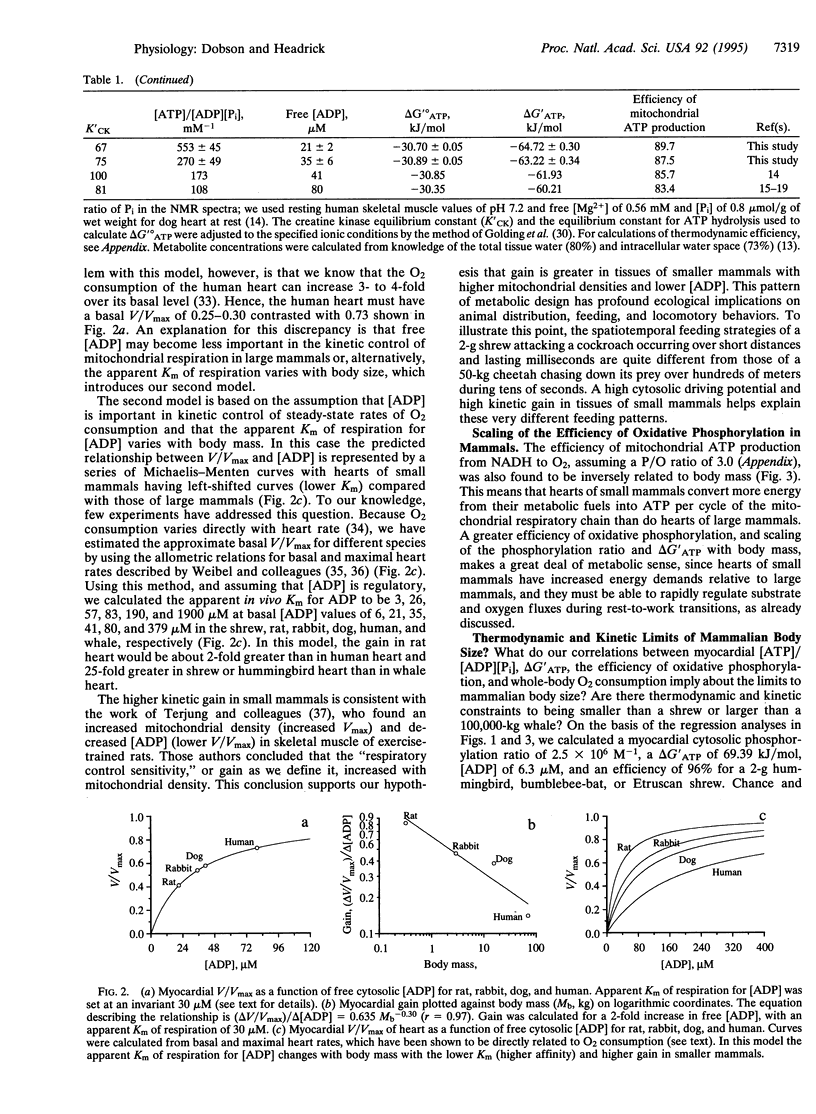
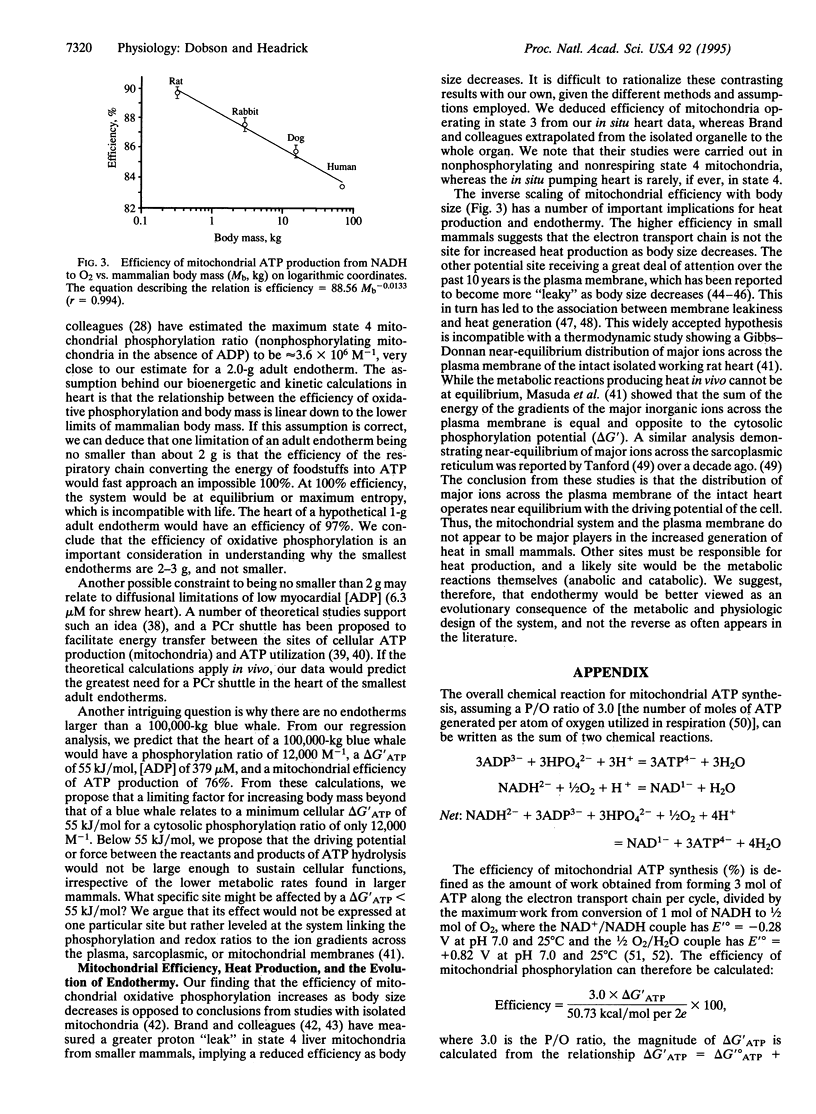
The cytosolic phosphorylation ratio ([ATP]/[ADP][P(i)]) in the mammalian heart was found to be inversely related to body mass with an exponent of -0.30 (r = 0.999). This exponent is similar to -0.25 calculated for the mass-specific O2 consumption. The inverse of cytosolic free [ADP], the Gibbs energy of ATP hydrolysis (delta G'ATP), and the efficiency of ATP production (energy captured in forming 3 mol of ATP per cycle along the mitochondrial respiratory chain from NADH to 1/2 O2) were all found to scale with body mass with a negative exponent. On the basis of scaling of the phosphorylation ratio and free cytosolic [ADP], we propose that the myocardium and other tissues of small mammals represent a metabolic system with a higher driving potential (a higher delta G'ATP from the higher [ATP]/[ADP][P(i)]) and a higher kinetic gain [(delta V/Vmax)/delta [ADP]] where small changes in free [ADP] produce large changes in steady-state rates of O2 consumption. From the inverse relationship between mitochondrial efficiency and body size we calculate that tissues of small mammals are more efficient than those of large mammals in converting energy from the oxidation of foodstuffs to the bond energy of ATP. A higher efficiency also indicates that mitochondrial electron transport is not the major site for higher heat production in small mammals. We further propose that the lower limit of about 2 g for adult endotherm body size (bumblebee-bat, Estrucan shrew, and hummingbird) may be set by the thermodynamics of the electron transport chain. The upper limit for body size (100,000-kg adult blue whale) may relate to a minimum delta G'ATP of approximately 55 kJ/mol for a cytoplasmic phosphorylation ratio of 12,000 M-1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth E., Stämmler G., Speiser B., Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992 Jul;24(7):669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- Bessman S. P. The creatine phosphate energy shuttle--the molecular asymmetry of a "pool". Anal Biochem. 1987 Mar;161(2):519–523. doi: 10.1016/0003-2697(87)90483-0. [DOI] [PubMed] [Google Scholar]
- Blackledge M. J., Rajagopalan B., Oberhaensli R. D., Bolas N. M., Styles P., Radda G. K. Quantitative studies of human cardiac metabolism by 31P rotating-frame NMR. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4283–4287. doi: 10.1073/pnas.84.12.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P. A., Hardy C. J., Roemer P. B. Phosphate metabolite imaging and concentration measurements in human heart by nuclear magnetic resonance. Magn Reson Med. 1990 Jun;14(3):425–434. doi: 10.1002/mrm.1910140302. [DOI] [PubMed] [Google Scholar]
- Bottomley P. A. Noninvasive study of high-energy phosphate metabolism in human heart by depth-resolved 31P NMR spectroscopy. Science. 1985 Aug 23;229(4715):769–772. doi: 10.1126/science.4023711. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Chien L. F., Ainscow E. K., Rolfe D. F., Porter R. K. The causes and functions of mitochondrial proton leak. Biochim Biophys Acta. 1994 Aug 30;1187(2):132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Cadoux-Hudson T. A., Blackledge M. J., Radda G. K. Imaging of human brain creatine kinase activity in vivo. FASEB J. 1989 Dec;3(14):2660–2666. doi: 10.1096/fasebj.3.14.2629743. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Clark B. J., Maris J., Kent J., Nioka S., Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Kent J., McCully K., Nioka S., Clark B. J., Maris J. M., Graham T. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9458–9462. doi: 10.1073/pnas.83.24.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE FRANCISCIS G., FUTRELL M. F., KUNKEL H. O., SPALDING J. F. Cytochrome oxidase activity and body weight in rats and in three species of large animals. Am J Physiol. 1956 Aug;186(2):203–206. doi: 10.1152/ajplegacy.1956.186.2.203. [DOI] [PubMed] [Google Scholar]
- Dudley G. A., Tullson P. C., Terjung R. L. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem. 1987 Jul 5;262(19):9109–9114. [PubMed] [Google Scholar]
- Emmett B., Hochachka P. W. Scaling of oxidative and glycolytic enzymes in mammals. Respir Physiol. 1981 Sep;45(3):261–272. doi: 10.1016/0034-5687(81)90010-4. [DOI] [PubMed] [Google Scholar]
- Feldman H. A., McMahon T. A. The 3/4 mass exponent for energy metabolism is not a statistical artifact. Respir Physiol. 1983 May;52(2):149–163. doi: 10.1016/0034-5687(83)90002-6. [DOI] [PubMed] [Google Scholar]
- Golding E. M., Teague W. E., Jr, Dobson G. P. Adjustment of K' to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol. 1995 Aug;198(Pt 8):1775–1782. doi: 10.1242/jeb.198.8.1775. [DOI] [PubMed] [Google Scholar]
- Gyulai L., Roth Z., Leigh J. S., Jr, Chance B. Bioenergetic studies of mitochondrial oxidative phosphorylation using 31phosphorus NMR. J Biol Chem. 1985 Apr 10;260(7):3947–3954. [PubMed] [Google Scholar]
- Headrick J. P., Dobson G. P., Williams J. P., McKirdy J. C., Jordan L., Willis R. J. Bioenergetics and control of oxygen consumption in the in situ rat heart. Am J Physiol. 1994 Sep;267(3 Pt 2):H1074–H1084. doi: 10.1152/ajpheart.1994.267.3.H1074. [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Lindstedt S. L., Claassen H., Taylor C. R., Mathieu O., Weibel E. R. Scaling mitochondrial volume in heart to body mass. Respir Physiol. 1984 Feb;55(2):131–137. doi: 10.1016/0034-5687(84)90018-5. [DOI] [PubMed] [Google Scholar]
- Hulbert A. J., Else P. L. Evolution of mammalian endothermic metabolism: mitochondrial activity and cell composition. Am J Physiol. 1989 Jan;256(1 Pt 2):R63–R69. doi: 10.1152/ajpregu.1989.256.1.R63. [DOI] [PubMed] [Google Scholar]
- JANSKY L. Total cytochrome oxidase activity and its relation to basal and maximal metabolism. Nature. 1961 Mar 18;189:921–922. doi: 10.1038/189921a0. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E. Theoretical support for the heart phosphocreatine energy transport shuttle based on the intracellular diffusion limited mobility of ADP. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1035–1041. doi: 10.1016/0006-291x(85)91240-9. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A. Lethal myocardial ischemic injury. Am J Pathol. 1981 Feb;102(2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Katz L. A., Swain J. A., Portman M. A., Balaban R. S. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol. 1989 Jan;256(1 Pt 2):H265–H274. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- Masuda T., Dobson G. P., Veech R. L. The Gibbs-Donnan near-equilibrium system of heart. J Biol Chem. 1990 Nov 25;265(33):20321–20334. [PubMed] [Google Scholar]
- Mathieu O., Krauer R., Hoppeler H., Gehr P., Lindstedt S. L., Alexander R. M., Taylor C. R., Weibel E. R. Design of the mammalian respiratory system. VII. Scaling mitochondrial volume in skeletal muscle to body mass. Respir Physiol. 1981 Apr;44(1):113–128. doi: 10.1016/0034-5687(81)90079-7. [DOI] [PubMed] [Google Scholar]
- Porter R. K., Brand M. D. Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate. Nature. 1993 Apr 15;362(6421):628–630. doi: 10.1038/362628a0. [DOI] [PubMed] [Google Scholar]
- Portman M. A., Heineman F. W., Balaban R. S. Developmental changes in the relation between phosphate metabolites and oxygen consumption in the sheep heart in vivo. J Clin Invest. 1989 Feb;83(2):456–464. doi: 10.1172/JCI113904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben J. The evolution of endothermy in mammals and birds: from physiology to fossils. Annu Rev Physiol. 1995;57:69–95. doi: 10.1146/annurev.ph.57.030195.000441. [DOI] [PubMed] [Google Scholar]
- Schaefer S., Schwartz G. G., Steinman S. K., Meyerhoff D. J., Massie B. M., Weiner M. W. Metabolic response of the human heart to inotropic stimulation: in vivo phosphorus-31 studies of normal and cardiomyopathic myocardium. Magn Reson Med. 1992 Jun;25(2):260–272. doi: 10.1002/mrm.1910250205. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Briggs R. W., Radda G. K. 31p NMR saturation transfer measurements of the steady state rates of creatine kinase and ATP synthetase in the rat brain. FEBS Lett. 1982 Apr 19;140(2):289–292. doi: 10.1016/0014-5793(82)80916-2. [DOI] [PubMed] [Google Scholar]
- Stahl W. R. Scaling of respiratory variables in mammals. J Appl Physiol. 1967 Mar;22(3):453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- Tanford C. Equilibrium state of ATP-driven ion pumps in relation to physiological ion concentration gradients. J Gen Physiol. 1981 Feb;77(2):223–229. doi: 10.1085/jgp.77.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Taylor C. R., Hoppeler H. Variations in function and design: testing symmorphosis in the respiratory system. Respir Physiol. 1992 Mar;87(3):325–348. doi: 10.1016/0034-5687(92)90015-o. [DOI] [PubMed] [Google Scholar]
- Wyss M., Smeitink J., Wevers R. A., Wallimann T. Mitochondrial creatine kinase: a key enzyme of aerobic energy metabolism. Biochim Biophys Acta. 1992 Sep 25;1102(2):119–166. doi: 10.1016/0005-2728(92)90096-k. [DOI] [PubMed] [Google Scholar]


