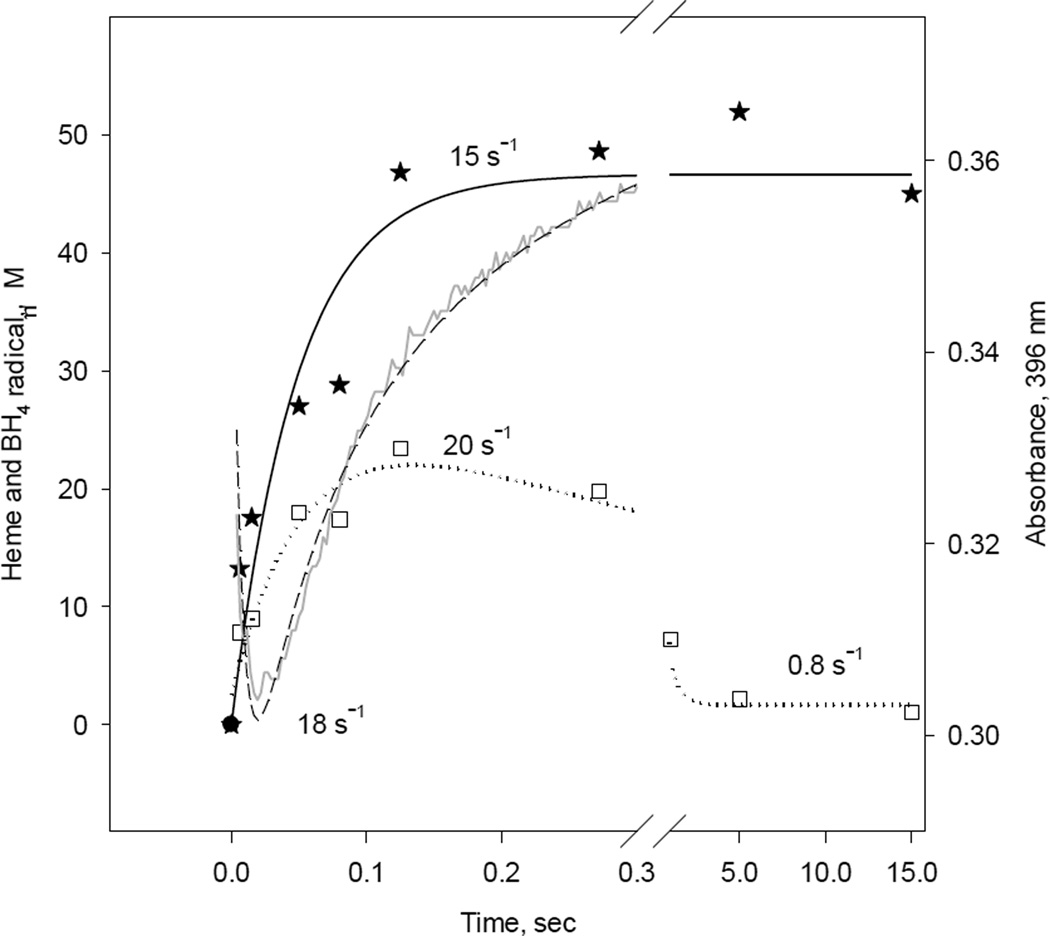
Fig. 10. Kinetic correlation between heme redox changes and BH4 radical during reaction of reduced iNOSox(+BH4, +L-arg) with oxygen.
Single wavelength stopped-flow at 396 nm obtained during reaction of 10 µM iNOSox(+BH4, +L-arg) with an air-saturated buffer (dash line) at 22 °C and parallel RFQ EPR kinetics of 50 µM protein to obtain amplitudes of BH4 radical, g= 2 signal (square), and high spin ferric heme, g= 7.8 signal (star) recorded at 10K. The lines and corresponding rate constants were obtained by a one- exponential fit for the g= 7.8 signal and an irreversible A→B→C sequential mechanism for g= 2 signal and A↔B→C for A396 stopped-flow data.