Figure 5. The uninduced state of the PTC and an unaccommodated A-tRNA in the ErmBL-SRC prevent peptide bond formation.
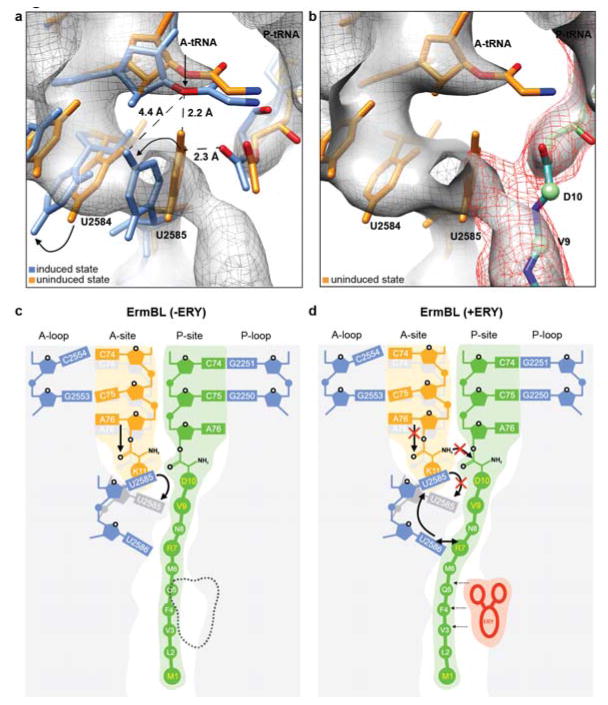
a-b, During accommodation of the A-tRNA, U2585 and U2584 undergo conformational changes (arrowed) necessary for peptide bond formation21-23. In the ErmBL-SRC, the electron density suggests that U2585 retains an unaccommodated state (orange, PDB1VQ6)21-23 thus preventing proper placement of the A-tRNA in the PTC (blue, PDB1VQN)21-23. b, Electron density between the ErmBL nascent chain (dark mesh) and U2585 indicates that the C-terminal amino acids of ErmBL directly interact with U2585, likely restricting its movement. c, d, Model for the translation of ErmBL (c) in the absence of erythromycin (canonical translation), and (d) translation arrest in the presence of erythromycin (stalling). In (d), the drug restricts the conformational space available for the ErmBL nascent chain such that interactions with U2586 and U2585 are established. We propose that this prevents movement of U2585 from the uninduced to induced state and thus, hinders accommodation of the A-tRNA and peptide bond formation.
