Figure 2. Chemical Structures of (S)-ketamine and Phencyclidine.
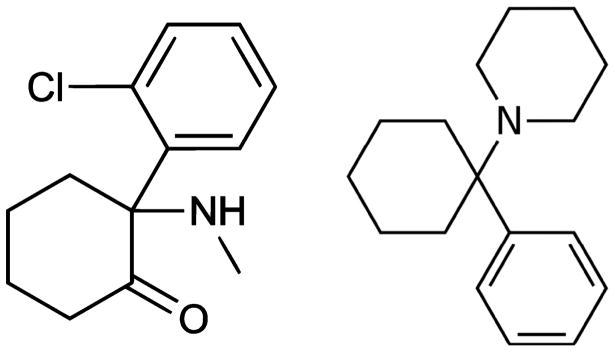
(S)-ketamine, left, is the more potent of ketamine’s two enantiomers. It is known as 2-chlorphenyl-2-methylamino-cyclohexanone by International Union of Pure and Applied Chemists (IUPAC) nomenclature. Phencyclidine (PCP, right) and ketamine share a binding site within the pore of the NMDAR and induce similar effects. Both chemicals are dissociative anesthetics and share structural similarities such as aromaticity.
