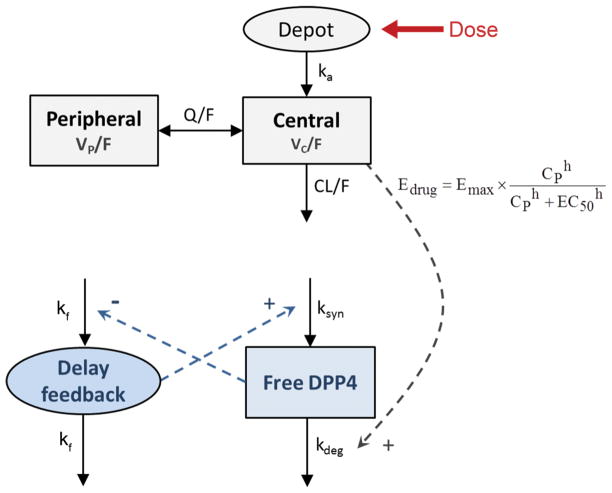
Fig. 1.
Schematic representation of the selected pharmacokinetic/pharmacodynamic model. Pharmacokinetics: ka, first order rate constant of absorption; VC/F, and VP/F, apparent volumes of distribution of the central and the peripheral compartments, respectively; CL/F apparent plasma clearance (oral); Q/F, inter-compartmental distribution clearance. pharmacodynamics: DPP4 activity (free receptor) can be synthesized at a zero-order rate (ksyn) and degraded at a first-order (kdeg). Edrug induces activity loss by the receptor binding. CP, predicted sitagliptin plasma concentration; Emax is the maximum effect; EC50 is the drug concentration need to achieve the 50% of the Emax; h is the hill exponent. 1/kf represents the expected time delay for the feedback.