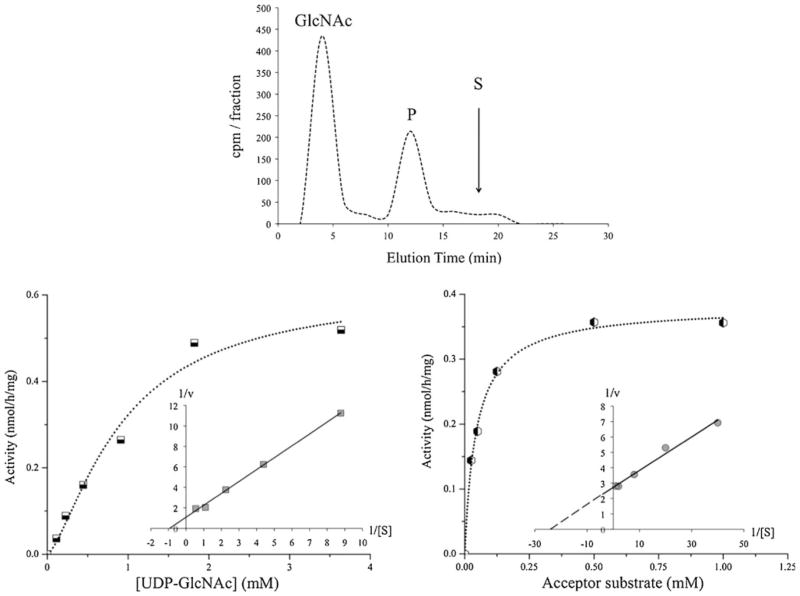
Fig. 1.
β1,6-N-acetylglucosaminyltransferase Venzymatic activity and kinetics. GnT-V enzymatic activity in homogenates from PC-3 cells (derived from bone metastasis) was measured as described in Materials and Methods. GnT-V enzymatic activity was found to be increased in all prostatic cancer cells compared to normal prostatic cells. a Enzyme reaction product was separated by HPLC using 24 % acetonitrile in H2O as the mobile phase. The radioactivity (cpm) of collected 2 min fractions is shown. The elution of radioactive GlcNAc and specific acceptor substrate (S), GlcNAcβ2Manα6(GlcNAcβ2[4-deoxy-]Manα3)[4-O-methyl-]Manβ-octyl, is indicated by the arrow; this was well separated from the radioactive enzyme product (P). b The apparent KM for UDP-GlcNAc was 0.93 mM and Vmax was 0.62 nmol/h/mg (with 0.5 mM acceptor substrate). The inset shows the Lineweaver-Burk plot. c The apparent KM for the acceptor substrate was 0.05 mM and the apparent Vmax was 0.38 nmol/h/mg (with 1 mM UDP-GlcNAc). The inset shows the Lineweaver-Burk plot