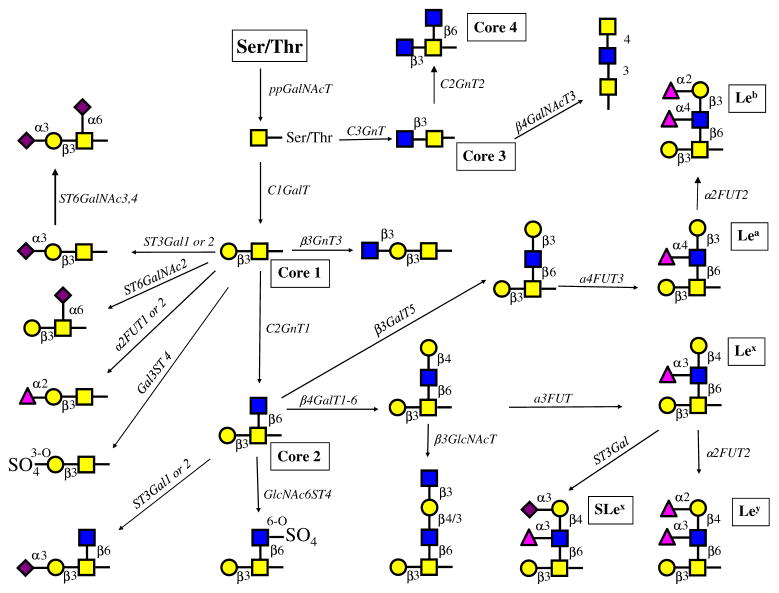
Fig. 3.
Proposed major O-glycosylation pathways in normal and prostatic cancer cells. Based on the major enzymatic activities and the relative gene expression levels of glycosyltransferases and sulfotransferases, the proposed major O-glycosylation pathways in prostate cells were constructed (nomenclature is shown in Table 1). Many other minor pathways are possible. Real-time PCR showed the expression of isoenzymes. RWPE-1 cells can synthesize core 1, 2 and 3 structures. Which can be extended and modified by the addition of sugar residues or sulfate esters and Lewis antigens Lea, Leb, Lex, Ley and SLex Only RPWE-1 cells showed the activity that synthesizes core 3 but not the activity that synthesizes core 4 although the enzyme (C2GnT2) was expressed at low levels. Both PC-3 (from bone metastasis) and DU145 (from brain metastasis) lack C2GnT1 activity and GCNT1 expression, which results in shorter O-glycan chains with mainly sialylated core 1 structures. DU145 cells exhibit a low ST3Gal enzymatic activity, whereas PC-3 cells exhibit a high enzymatic activity and a high gene expression of ST3GAL1. The prostatic cancer cells appear to be unable to synthesize core structures 3 and 4 although significant amounts of mRNA for the respective enzyme genes were found. LNCaP cells (from lymph node metastasis) contain high enzymatic C2GnT activity and gene expression level of GCNT1 and can form complex core 1 and core 2, but not core 3 O-glycans. ST3Gal enzymatic activity and gene expression are high in LNCaP cells. These glycans can be modified to form a variety of complex structures with extension enzymes as well as α1,2-and α1,3FUT4 and Gal3ST, but not α3/4FUT3. Although the activities of α2,6-sialyltransferases were not directly measured, the gene expression levels suggest that sialylα2-6GalNAc- and sialylated core 1 structures can be synthesized. Sulfotransferases using GlcNAc-R substrate are expressed although the activities were not detected. However, sulfotransferases using core 1 substrate were active. VCaP cells differ from LNCaP in that the expression of sulfotransferases Gal3ST1-4 and the enzymatic activity of Gal3ST is also higher in VCaP cells. α3/4FUT3 and α3FUT4 are active in VCaP cells and can synthesize various Lewis antigens