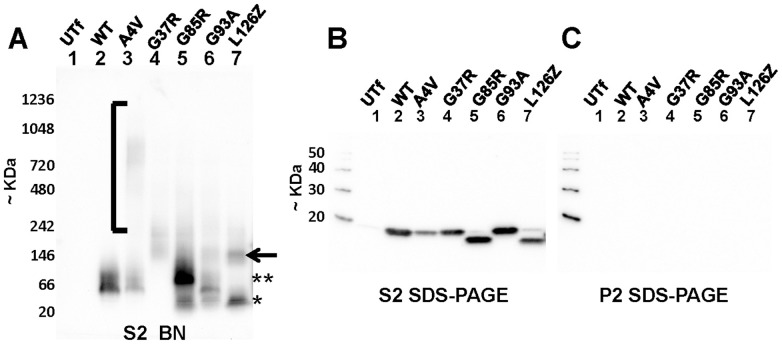
Figure 2. Immunoblots of BN-gels analyzing the electrophoretic mobility of WT and mutant SOD1.
As described in Methods and diagrammed in Fig. 1, HEK293FT cells were transiently transfected with vectors to express WT or mutant SOD1. 24 hours post-transfection, the cells were lysed in digitonin and then soluble fractions were subjected to high speed centrifugation to generate an S2 soluble fraction and a P2 pellet fraction. (A) 16 µl of S2 soluble fraction was analyzed by BNGE and immunoblotting with hSOD1 antibody (which recognizes aa 24–36 of human SOD1 [32]). In this experiment, the PDVF membrane was air dried to fix proteins to the membrane. (B and C) To estimate loading and assess the level of large aggregates in the S1 fractions, 16 µl of the soluble fraction and 1/10 of total P2 fraction (roughly equivalent volumetrically to what is analyzed for the S2 fraction) were analyzed by SDS-PAGE and immunoblotting with the hSOD1 antibody. The membranes for each of the SDS-PAGE blots were incubated with primary and secondary antibodies in parallel (antibody dilutions prepared from the same stock and portioned out from a single mixture) and imaged simultaneously. The images shown are representative of at least 3 independent replications.