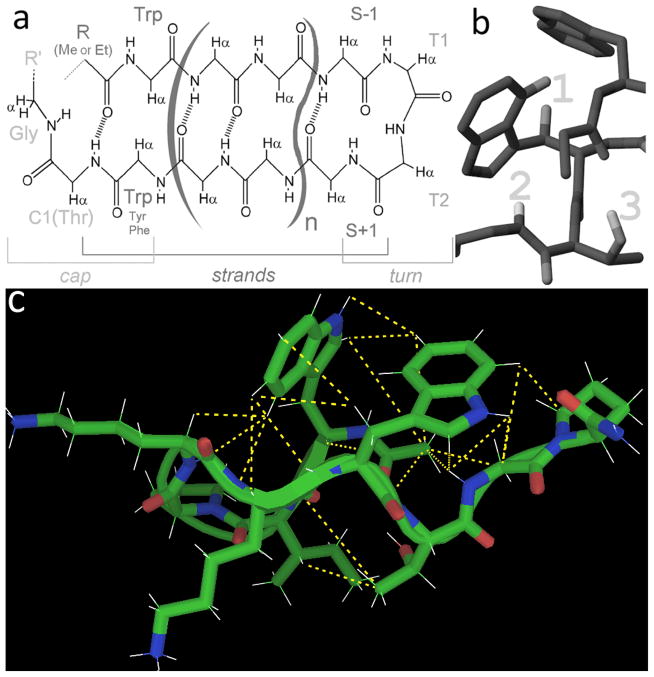
Figure 1.
The beta cap can be used on any length hairpin and maintain the same conformation. (a: the archetypal example; “n” can be any number, but must include two amino acids per strand for proper alignment. b) Key features of the beta cap, including the edge-to-face Trp/Trp interaction (upfield protons at 1) a Gly amide→Trp indole H-bond (upfield proton 2) and a bifurcated H-bond involving the N-terminal carbonyl and the Thr sidechain (Hγ1 proton visible by NMR in aqueous media, 3) c) the generic, N-acetylated form of the captide used in this study, shown with structure-validating “long”-range (i−i+>1) NOEs. These same NOEs are observed for all well-folded captides and serve as further confirmation of structure.